


Pinnita Prabhasawat
Application of Preserved Human Amniotic Membrane for Corneal Surface Reconstruction
Introduction
Human amniotic membrane has been used for many purposes since 19101. It has been widely used as an effective biological dressing for acute burn2-5 , skin ulcers6-7 and abdominal wound8 to relieve pain, promote epithelial healing and decrease infection rate of the wound. The amniotic membrane also is used as a graft for burned skin9-10, vaginal reconstruction in the absence of vagina11-12, reconstruction in otolarynx13, and repair omphalocele14. Successful use of amniotic membrane to prevent tissue adhesion in surgeries of the head, abdominal and pelvic cavity15-17 has also been reported.
In Ophthalmology, human amniotic membrane has been used as grafts for pterygium18 and conjunctival defect19 since 1940. Since 1993, preserved human amniotic membrane has been used actively; the first report of its effectiveness was in the reconstruction of corneal surface in rabbit eye by Kim and Tseng20 . Since then the membrane has been widely used successfully for many purposes in the ocular surface. It has been shown to be effective in promoting epithelial healing in persistent epithelial defect21, and in combination with limbal transplantation in Steven Johnson’s syndrome and chemical burn22-24. Its used as an alternative graft in pterygium excision25-26, symblepharon and conjunctival tumors27 has also been reported as well as in the prevention of fibrosis in trabeculectomy28 and haze reduction in PRK29-30. Ocular surface disorders are caused by many conditions of the cornea and conjunctiva and there are still many problems and difficulties in treatment. Persistent corneal epithelial defect, or irregular surface of the cornea from bullous or band keratopathy can cause not only irritation and pain to the patient but also reduce protective mechanism and lead to get the infection of the cornea. The conventional treatment such as conjunctival flap, contact lens or tarsorrhaphy can promote epithelial healing and relieve pain. However, conjunctival flap will change the clear cornea to vascularization. Contact lens may increase risk of infection and not be effective in some cases and tarsorrhaphy affects the appearance of the patient. In limbal deficiency patients in the past there has been no conventional treatment and a successful method of treatment is still being sought. Lysis adhesion from the scar and symblepharon or wide excision of the conjunctival mass still needs a good graft in terms of ease of application, good cosmetic results, and prevention of tissue adhesion. Because the results of amniotic membrane transplantation are encouraging and treatment of ocular surface disorders still has many problem to solve, we would like to examine the efficacy of using preserved human amniotic membrane for ocular surface problems. We divided the purposes for ocular surface disorders into 2 main groups, the first is for corneal surface reconstruction and the second is for conjunctival surface reconstruction. The details of indications for surgery and the outcome have been described.
Title : Application of Preserved Human Amniotic Membrane for Corneal Surface Reconstruction
Pinnita Prabhasawat
Pinnita Prabhasawat, MD, Department of Ophthalmology, Siriraj Hospital, Faculty of Medicine, Mahidol University, 2 Prannok Road, Bangkoknoi, Bangkok 10700, Thailand.
Tel: (662) 411-2006 Fax: (662) 411-1906 E-mail: sippb@mahidol.ac.th
Supported in part by the grant of Faculty of Medicine, Siriraj Hospital
Presented in part at the 7th International Conference of the Asia Pacific Association of Surgical Tissue Banking, Kuala Lumpur, Malaysia, November 1998, in part at the Thai-Japan Joint Meeting, Bangkok, Thailand, January 1999 and in part at the fourth Annual Meeting of the Ocular Surface and Tear Conference, Miami, Florida, May 1999.
ABSTRACT
Objective: To evaluate the efficacy of preserved human amniotic membrane transplantation for reconstruction of the corneal surface diseases.
Methods: Preserved human amniotic membrane transplantations were performed in 84 eyes of 78 patients for corneal surface reconstruction. The indications were limbal stem cell deficiency from Steven-Johnson syndrome, chemical burn and herpes keratitis (27 eyes), bullous keratopathy (26 eyes), persistent epithelial defect and dellen (17 eyes), band keratopathy (11 eyes), preparing for prosthesis (1 eye), corneal ulcer (1 eye) and acute chemical burn (1 eye).
Results: Success was noted in 83.3% (70/84) eyes, partial success in 13.1% (11/84) eyes, and failure in 3.6% (3/84) eyes for an average follow-up of 10.5 months (3 to 29 months). No patient developed major immediate post-operative complications.
Conclusion: Amniotic membrane transplantation can reduce inflammation, promote corneal epithelial healing, and decrease irritation in corneal surface problems.
Abbreviations
AMG = Amniotic membrane graft
AMT = Amniotic membrane transplantation
EBSS = Eale’s balanced salt solution
ED = Epithelial defect
EDTA = Ethylene diamine tetrachloroacetic acid
LT = Limbal transplantation
PED = Persistent epithelial defect
PKP = Penetrating keratoplasty
PO = Punctal occlusion
INTRODUCTION
Since 1910, human amniotic membrane has been used for many purposes. (Trelford et al.1979, p.833-45). Both fresh and preserved human amniotic membrane have been widely used effectively as a biological dressing for acute burns (Bose 1979; Sharma et al. 1985; Talmi et al. 1990; Ramakrishnan et al. 1997) , skin ulcers (Somerville 1982; Shun et al. 1983) and abdominal wounds (Silverton et al. 1979) to relieve pain, promote epithelial healing and decrease infection rate of the wound. The amniotic membrane has also been used as a graft for burned skin (Waikakul et al. 1990; Subrahmanyam 1995), vaginal reconstruction in the absence of vagina (Dhall 1984; Bleggi-Torres et al. 1997), reconstruction in otolarynx (Zohar et al. 1987), and repair omphalocele (Yokomari et al. 1992). It has also been reported to prevent tissue adhesion in surgeries of head, abdominal and pelvic cavity (Trelford-Sauder et al. 1978; Young et al 1991; Rennekampff et al. 1994).
Human amniotic membrane was first used as a graft for treating conjunctival defects (DeRoth 1940, p.522-5) and pterygium (Panzardi 1947, p.332-6) since 1940. In 1993, preserved human amniotic membrane was successfully used in corneal surface reconstruction in rabbit eyes by Kim and Tseng (1995). Since then, this technique has been applied as an adjunct in the treatment of many ocular surface disorders. In corneal surface reconstruction, the amniotic membrane is reported to have been used in persistent epithelial defects (Lee et al. 1997; Kruse et al. 1999; Azuara-Blanco et al 1999), combined with limbal transplantation in Steven-Johnson syndrome, and chemical burns (Tsubota et al 1996; Shimazaki et al. 1997; Tseng et al. 1998; Tsubota et al 1999), bullous keratopathy (Pires et al. 1999) and for reducing haze in following phototherapeutic keratectomy (PRK) (Wang et al. 1997; Choi et al. 1998).
An ideal graft for use in ocular surface reconstruction would not only, to a maximum, promote healing while minimizing scarring, but would also yield cosmetically acceptable results and be relatively easy to perform. Human amniotic membrane clearly has shown promise along these lines. To further investigate its applicability, we have used this technique for corneal surface reconstruction in the treatment of several severe eye conditions encountered in a Bangkok public hospital.
PATIENTS AND METHODS
Patients
This study was approved by the committee for protection of human subjects in research at the Faculty of Medicine, Siriraj Hospital, Mahidol University. From September, 1997 to March, 2000, amniotic membrane transplantation was performed in 78 patients (84 eyes) with various corneal surface disorders after obtaining their informed consents. Their clinical data, indications for surgeries and goal of treatments in each group are summarized in Tables 1 and 2.
The patients were divided into 2 groups depending on the indications. The first group of 21 cases (27 eyes) showed limbal stem cell deficiency, caused by Steven-Johnson disease (13 cases, 18 eyes), chemical burn (7 cases, 8 eyes), and HSV keratitis (1 case, 1 eye). In this group, 12 patients (12 eyes) received amniotic membrane transplantation (AMT) combined with limbal transplantation in the same or subsequent operation, of which 7 patients (7 eyes) received autograft and 5 patients (5 eyes) received allograft limbal transplantation. Nine patients (13 eyes) received AMT alone because of their financial difficulties of obtaining systemic cyclosporin which is indispensable for allograft limbal transplantation. The second group consisted of other corneal diseases such as bullous keratopathy (26 cases, 26 eyes), persistent epithelial defect, dellen and descemetocele (17 cases, 17 eyes), band keratopathy after removal of calcium deposit (11 cases, 11 eyes), preparing for prosthesis (1 case, 1 eye), corneal ulcer (1 case, 1 eye), and acute chemical burn (1 case, 1 eye).
Preparation of preserved human amniotic membrane
Human placentas were obtained during elective caesarian sections with who were seronegative for human immunodeficiency virus (HIV) (antigen and antibody), hepatitis B and C viruses, and syphilis. Placentas were kept in sterile plastic bags on ice during transfer to the tissue bank of Bangkok, and were handled at all times using sterile technique. Under a lamellar-flow hood, placentas were rinsed several times, to remove excessive blood clots, with 0.9% normal saline containing 50 mg/ml penicillin, 50 mg/ml streptomycin, 100 mg/ml neomycin, and 2.5 mg/ml amphotericin B. With two sets of forceps, the amniotic membranes were separated from the remaining chorion by blunt dissection while immersed in the above antibiotics-containing Eale’s balanced salt solution (EBSS). The separated amniotic membranes were then apposed onto sterile nitrocellulose filters so that the epithelial surfaces were facing up when flattened. These sheets of amniotic membranes were then stored in 50% DMEM media and 50% glycerol mixed with the above antibiotics at –700C for up to one year before use. The procedures were identical to what has been reported (Lee et al. 1997; Tseng et al 1998; Prabhasawat et al. 1997) except normal saline was used instead of EBSS to rinse the blood clot.
Amniotic membrane transplantation
After obtaining the informed consent, the patient was subjected to the same routine admitting preoperative tests. One surgeon (PP) performed all surgeries using different techniques depending on the indications.
Amniotic membrane transplantation for limbal deficiency
After retrobulbar block with 2% xylocain combined with 2% marcain or general anesthesia, the abnormal scar and symblepharon was excised at the bulbar part area until the fornix. Amniotic membrane was covered on the whole surface area of the bulbar part and the corneal surface. Running suture with 10.0 nylon was performed at the limbal area to fix the amniotic membrane to the sclera. The interrupted suture with 8.0 vicryl or continuous running suture 10.0 nylon was performed to fix amniotic membrane to the sclera 5-7 mm away from the limbal area. The procedure was similar to that described by Tseng et al. (Tseng et al. 1998).
Amniotic membrane transplantation for bullous keratopathy and band keratopathy
After topical anesthesia with 2% tetracain eye drop, the loose epithelium was removed with a blade in bullous keratopathy or epithelium and calcium deposit was removed with 3% EDTA in band keratopathy. The amniotic membrane was placed to cover the corneal epithelial defect, which in general was up to 9 mm in diameter and close to the limbus. Running suture was performed near the edge of the membrane to ensure the tight approximation of the membrane to the denuded corneal surface. Interrupted sutures were added, if needed. A bandage lens was applied in some cases until the epithelial defect was healed.
Amniotic membrane transplantation for persistent epithelial defect, dellen, acute burn, and corneal ulcer
After retrobulbar injection or topical anesthetic drop (2% tetracaine eye drop), the base of the epithelial defect was debrided and covered with a piece of amniotic membrane sutured to the edge of the defect with interrupted 10-0 nylon. A bandage lens was applied until the defect was healed. The procedure was similar to that previously described (Lee et al. 1997).
Postoperative evaluation and follow up
After surgery, all patients were examined on the following day and subsequently daily while staying in hospital. After discharge, they were followed up at intervals of 1 week, 2 weeks and then monthly thereafter, or as needed for as long as possible. The same postoperative evaluation was applied to all patients with special attentions given to patient’s symptoms with respect to pain, discomfort, irritation, signs of inflammation at the surgical sites, and healing of the area covered by amniotic membrane using fluorescein staining. The outcome of AMT was judged by patient’s symptoms, resolution of original corneal surface problems, appearance of surgical sites, and recurrence of the diseases. Complications were recorded for infection, dislodgment or dissolution of the membrane graft with or without a non-healing ulcer or increased inflammatory and/or immune response over the membrane graft. If identified as the inciting cause but untreatable, the membrane was removed. Sterility of the membrane was rechecked again by bacterial and fungal cultures performed at the time following the surgery. All cultures were negative.
Clinical outcome was categorized into success, partial success, and failure based on the criteria as in Table 1. When all goals of surgery were achieved in terms of relief of pain and irritation, reduction of inflammation and scaring and promotion of epithelial healing, the outcome was defined as "success”. When part of these goals were achieved, e.g., relief of pain but still some inflammation or recurrence of the pain symptom exist, the outcome was defined as "partial success". When none of the surgical goals was met, the outcome was defined as "failure".
RESULTS
The average age of these 78 patients (42 men, 36 women) was 46.6 years (ranging from 3 to 85 years), and the average follow-up time was 10.5 months (ranging from 3 to 29 months). Based on the criteria given in Table 1, success, partial success and failure was obtained in 70 eyes (83.3 %), 11 eyes (13.1%) and 3 eyes (3.6%), respectively, Details of the success rate in each group are as shown in Table 2.
In limbal stem cell deficiency patients, 18 out of 27 eyes (66.7%) were rated as success, and these patients felt more comfortable without irritation or inflammation, and showed improved vision. The visions improved from hand motion to at least 20/200 in 4 eyes of AMT combined with limbal allograft transplantation and in 5 eyes of AMT combined with limbal autograft transplantaion. The vision improved from hand motion to at least 10/200 or more than 2 snellen lines in 6 eyes of AMT alone (Table 3). Seven out of 21 eyes (25.9%) were rated as partial success, with decreased inflammation, but mild fibrovascular tissue invasion into the grafts or slight recurrence of symblepharon. Importantly, all these patients with partial success felt more comfortable and satisfied with the outcome to the extent they would like to have the other eye operated. Two eyes (7.4%) were rated as failure. One of them showed recurrent conjunctival keratinization due to severe keratoconjunctivitis sicca. The other case still showed the same inflammation as before surgery. One eye showed corneal graft rejection after 9 months post triple surgery, combined amniotic membrane, limbal and corneal transplantation, however amniotic membrane graft still being quiet and indicating no sign of rejection. Intriguingly, all cases rated as partial success or failure suffered from Steven-Johnson syndrome.
In painful bullous keratopathy, 24 out of 26 eyes were rated as success and became pain-free with a stable corneal surface after AMT. The partial success cases were pseudophacic and traumatic bullous keratopathy. In both cases, pain and irritation were relieved in the first 1.5 and 2 months after surgery, but recurred thereafter, and required the use of contact lens to relieve pain and irritation. Nine out of eleven eyes with band keratopathy, which were rated as success, felt much more comfortable without irritation or pain and no recurrence after removal of calcific band keratopathy with 3% EDTA and the whole cornea covered with the amniotic membrane. Two patients were rated as partial success due to recurrence of band keratopathy at postoperative 5 months and 7 months, respectively, but they did not have any irritation and remained pain-free.
All 17 cases with persistent epithelial defect, dellen or descemetocele were rated success and their corneal surfaces were healed after AMT. The causes for their surface breakdowns were diverse and included herpes simplex keratitis, corneal ulcer, lid abnormalities from tumor removal, corneal surface irregularity, post radiation, following corneal graft rejection and post phacoemulsification. The average duration for healing epithelial defects was 2.0 weeks (ranging from 1 to 4 weeks). Among them, 2 cases had failed with bandage contact lens treatment prior to the amniotic membrane graft. In the five cases with descemetocele and dellen, the corneal stromal thickness increased after the healing promoted by amniotic membrane graft.
In one case, which suffered from irritation and pain, that prevented this blind patient from wearing eye prosthesis, AMT was successful to eliminate such irritation and pain that he did not undergo conjunctival flap. In the case with superficial corneal ulcer following candidiasis superficial keratectomy and amniotic membrane graft successfully restored the corneal surface integrity. The epithelial defect and ulcer was completely healed in 1 week despite prior failure following medical treatments for more than 3 weeks. However, in one case with acute (2 weeks after burns) stage 4 burn with a 9 mm epithelial defect with severe ischemia, amniotic transplantation failed to decrease inflammation and to promote epithelial healing.
Complications
We divided complications into 2 major groups (Table 3). Immediate complications, i.e., those developing within 1 month of surgery, detachment of amniotic membrane (2 eyes), and conjunctivitis, lid abscess, and entropion (one eye). Despite of detachment of amniotic membrane, the epithelial defect healed rapidly in 1 week in the patient with bullous keratopathy so that the patient became pain-free. The eye, which developed conjunctivitis, lid abscess and entropion, was from a patient with Steven-Johnson syndrome as a result of bacterial infection and the membrane was detached and dissolved in that area. This complication was successfully treated with antibiotics and conjunctival flap to cover the bare sclera.
Late complications were those which developed after 1 month of surgery and the epithelial surface had healed completely. Three eyes developed corneal ulcer. The first case suffering from Steven-Johnson syndrome developed corneal ulcer in 1 month after surgery due to contamination of the eye drop. The second case with pseudophacic bullous keratopathy developed corneal ulcer in 2 months after surgery as a result of the dust in the eye. The third case, suffering from limbal deficiency and persistent corneal epithelial defect due to chemical burn, received autolimbal transplantation and amniotic membrane transplantation and penetrating keratoplasty 2 months later. These procedures resulted in a clear and stable corneal graft 3 months after penetrating keratoplasty, but developed bacterial corneal ulcer which was successfully treated with topical antibiotics. Three eyes developed minute corneal perforation in Steven-Johnson syndrome in 2 months, 2 months and 9 months, respectively. In these three eyes, corneal perforation was less than 1 mm in diameter without signs of infection and was successfully treated with glue and contact lens.
CASE EXAMPLES
Case 1 For partial limbal deficiency
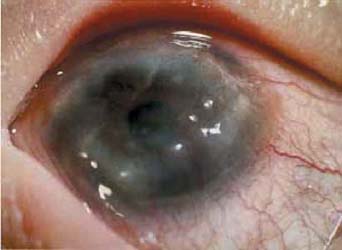
An HIV-positive 36 year-old male, diagnosed with Steven-Johnson syndrome and severe keratoconjunctivitis sicca, complained of painful irritation and inability of opening his eyes. The vision was 20/200 in both eyes, and the conjunctiva was marked injected in both eyes and symblepharon was noted in the temporal conjunctiva of the left eye (Fig.a). Despite occlusion of both upper and lower puncta, he remained symptomatic with marked inflammation. AMT was performed in both eyes in two settings to cover the whole cornea and bulbar conjunctiva 5 millimeters from the limbus as a graft. After surgery, his vision improved to 20/80, he felt much more comfortable, and there was no conjunctival injection in both eyes (Fig. b) at a follow-up of three months except at the previous symblepharon area in the left eye.
Case 2 For total limbal deficiency with combined allograft limbal transplantation.
A 50 year-old female suffered from irritation and blurred vision for many years due to limbal deficiency from herpes simplex keratitis and severe keratoconjunctivitis sicca in her right eye, for which she had received a penetrating keratoplasty 4 years ago. Her visual acuity in her right eye was counting finger at 2 ft. Moderate conjunctival injection was noted in both eyes, and all four puncta were occluded for treating severe keratoconjunctivitis sicca. Conjunctivalization was noted 3600 of her cornea (Fig. c). AMT was performed to cover the whole cornea and bulbar sclera 5-7 mm from the limbus in her right eye. After surgery, her eye had no irritation and the bulbar conjunctiva was smoothly wettable without inflammation. Three months later, allograft limbal transplantation was performed resulting in improved vision of 20/50, clear cornea without vascularization, and quiet conjunctiva (Fig. d) for a follow-up of 29 months.
Case 3 For bullous keratopathy
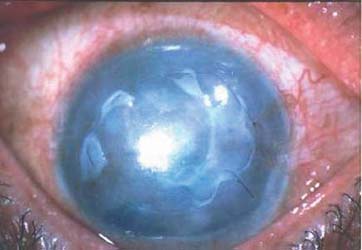
A 66 year-old female suffered from irritation and tearing in her right eye due to corneal decompensation following extracapsular cataract extraction and anterior chamber lens implantation 5 years ago. Her vision was hand motion, and the cornea was edematous with epithelial bullae (Fig. e). Following epithelial scraping and AMT, the epithelial defect healed in 1 week, and the transplanted amniotic membrane became transparent and difficult to be identified in one month (Fig. f). Eight months after surgery, her cornea remained edematous and bullae recurred, but she still enjoyed the comfort without irritation for the entire follow up of 18 months.
Case 4 For persistent epithelial defect
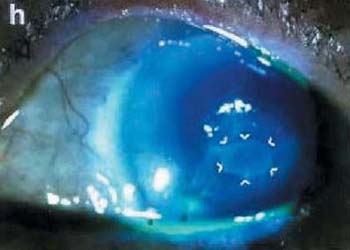
A one-eyed 87 year-old female developed persistent corneal epithelial defect from herpes simplex keratitis for more than 2 weeks, which was refractory to treatment including bandage contact lens and frequent tear substitutes. Her vision dropped to 3/200, and the defect showed progressive thinning (Fig.g). AMT was performed to cover the epithelial defect, which showed healing at postoperative day 6 and completed in 3 weeks. At that time, amniotic membrane partially dissolved, but still attached at the lesion (Fig.h) and totally dissolved after 2 months. Her vision improved to 20/50, the corneal stroma became thickening and the corneal epithelium did not show any recurrent breakdown for 15 months of follow-up.
DISCUSSION
From this result, we have shown that preserved human amniotic membrane is useful for difficult corneal surface problems (Table 2). This technique has achieved success rates of 83.3% and partial success rates of 13.1%. The usefulness and limitation of this procedure differ among different indications and are discussed below.
Loss of corneal stem cells due to destruction by external or internal injuries such as chemical burn, severe infection, and Steven-Johnson syndrome cause the limbal stem deficiency disease. Corneal surface gets conjunctivalization and vascularization. This can cause discomfort, photophobia, irritation, inflammation, decreased vision and recurrent corneal epithelial defect. It is a difficult disorder in which conventional penetrating keratoplasty has a limited role. Tseng (Tseng et al 1998) recently showed that amniotic membrane transplantation alone can be used to reconstruct corneal surfaces with partial limbal deficiency, i.e., there is part of the limbus retaining healthy limbal epithelial stem cells. In this study, when limbal deficiency is not severe, AMT alone can restore corneal surface smoothness and eliminate photophobia – e.g. case 1 - and improve the visual outcome. As shown by the result 6 eyes in the group of AMT alone had visual improvement - at least 2 snellen lines - even they still had corneal vascularization (Table 3). Furthermore, in this group, except 2 patients (7.4%), all others felt more comfortable, moist, less irritation, less keratinization and pleased with the result and asked to do the other eye as well. The reason why the patients feel more moist in their eyes and less keratinization might be because the amniotic membrane can increase goblet cell density of conjunctiva (Prabhasawat et al. 1997). Besides, cyclosporine is not required in AMT, which is a benefit for developing countries like Thailand for which the high expenses of cyclosporine represent a serious problem. However, when the limbal stem cell deficiency becomes total and diffuse, AMT alone is not sufficient (Tseng et al. 1998) and requires additional limbal stem cell transplantation for corneal surface reconstruction (Tsubota et al. 1996; Shimazaki et al. 1997; Tsubota et al. 1999). Here we also support this concept as all 5 eyes receiving AMT combined with limbal allografts and 5 eyes receiving AMT combined with limbal autograft had visual improvement and symptomatic relief while 9 eyes receiving AMT alone could not improve the vision.
In bullous keratopathy, the cornea is edema due to endothelial dysfunction. The corneal epithelium also becomes unhealthy and gets an irregular surface causing ocular pain and surface breakdown. This generally requires penetrating keratoplasty to relief the ocular discomfort and to improve vision. Lee (Lee et al. 1997) first reported that amniotic membrane can be used as an alternative to conjunctival graft to relief pain and recurrent erosion. We noted here amniotic membrane is an excellent alternative for countries like Thailand where there is a shortage of corneal donors, to relief pain and reduce surface inflammation while the patient is on the waiting list for corneal transplantation. Although the mechanism remains unclear, we are intrigued by the finding that symptomatic relief persists even there remains marked edema and recurrent bullae several months following AMT. This result is the same as the report of Pires (Pires et al. 1999).
When there is a persistent epithelial defect, corneal ulcer and perforation can develop. The conventional methods to treat the defect include the use of contact lens, conjunctival flap (Gundersen 1958; Insler et al. 1987) or tarsorrhaphy (Welch et al. 1988). Consistent with what has been reported by Lee (Lee et al. 1997) and Kruse (Kruse et al. 1999), our result also noted an overwhelming success of treating this indication. Surprisingly, AMT not only can promote epithelial healing, but also increase the corneal thickness in the case of dellen and descemetocele. This prevents corneal perforation, thus solving the surface healing problem refractory to medical treatments and contact lens wear. Furthermore, it also gave a good cosmetic result and improved vision (Case 4).
We did not note any sign of graft rejection, and no patient became clinically worse than before the surgery in term of visual acuity or inflammation. Only one case developed mild entropion after symblepharon lysis in Steven-Johnson syndrome, and this complication slowly resolved 2 months later without the need of surgical correction. Immediate complications were not serious and treatable. For example, bacterial conjunctivitis was successfully treated with topical antibiotics. The cause of infection was not directly linked to the preparation of amniotic membrane as microbial cultures of the amniotic membrane during preparation or following surgeries were all negative. Late complications may or may not be related to the procedure itself as they can be derived from the underlying disorders. For example late occurrence of corneal ulcer and perforation developed after the surface had healed. Furthermore, it is well known that diseases such as bullous keratopathy, penetrating keratoplasty and Steven-Johnson syndrome have weak ocular surface defense and are prone to develop corneal ulcer. Among all ocular surface disorders in this study, it is worth noting that Steven-Johnson syndrome is the disease which developed most complications and showed the lowest success.
In summary, this study has shown that the amniotic membrane can be used effectively to treat the corneal surface problems, ease to obtain and no need of immunosuppresive treatment like other organ transplantation. Future studies to understand more about the action of amniotic membrane transplantation may lead to other clinical uses.
ACKNOWLEDGMENTS
The authors wish to express their deep gratitude to Dr Scheffer C.G. Tseng for inspiration of using amniotic membrane and also thank the Department of Obstetric and Gynaecology for helpful cooperation to obtain the placenta, and the Bangkok Biomaterial Center for preparing and providing the amniotic membrane.
REFERENCES
Azuara-Blanco A, Pillai CT, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;83:399-402. |
|
Bleggi-Torres LF, Werner B, Piazza MJ. Ultrastructural study of the neovagina following the utilization of human amniotic membrane for treatment of congenital abscence of the vagina. Braz J Med Biol Res. 1997;3:861-864. |
|
Bose B. Skin wound dressing with human amniotic membrane. Ann R Coll Surg Eng. 1979;61:444-447. |
|
Choi YS, Kim JY. Wee WR, Lee JH. Effect of the application of human amniotic membrane on rabbit corneal wound healing after excimer laser photorefractive keratectomy. Cornea. 1998;17:389-395. |
|
DeRoth A. Plastic repair of conjunctival defects with fetal membranes. Arch Ophthalmol. 1940;23:522-525. |
|
Dhall K. Amnion graft for treatment of congenital abscence of the vagina. Br J Obstet Gynaecol. 1984;91:279-282. |
|
Gundersen T. Conjunctival flaps in the treatment of corneal disease with reference to a new technique of application. Arch Ophthalmol. 1958;60:880-888. |
|
Insler MS, Pechous B. Conjunctival flaps revisited. Ophthalmic Surg. 1987;18:455-458. |
|
Kim JC, Tseng SCG. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473-484. |
|
Kruse FE, Rohrschneider K, Volcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999;106:1504-1511. |
|
Lee S, Tseng SCG. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997; 123 :303-312. |
|
Panzardi D. Sul’uso delle membrane fetali come materiale per plastiche congiumtivali con paritcolare riguardo al loro impiego nello pterigio. Bulieltino D’Oculistica. 1947;26:332-336. |
|
Prabhasawat P, Tseng SCG. Impression cytology study of epithelial phenotype of ocular surface reconstructed by preserved human amniotic membrane. Arch Ophthalmol. 1997;115:1360-1367. |
|
Pires RTF, Tseng SCG, Prabhasawat P, Puangsricharern V, Maskin SL, Kim JC, Tan DTH. Amniotic membrane transplantation for symptomatic bullous keratopathy. Arch Ophthalmol. 1999: 117:1291-1297. |
|
Ramakrishnan KM, Jayaraman V. Management of partial-thickness burn wounds by amniotic membrane: a cost-effective treatment in developing countries. Burns. 1997;23 suppl:S33-36. |
|
Rennekampff HO, Dohrmann P, Fory R, Fandrich F. Evaluation of amniotic membrane as adhesion prophylaxis in a novel surgical gastroschisis model. J Invest Surg. 1994;7:187-193. |
|
Sharma SC, Bagree NM, Bhat AL, Banga BB, Singh MP. Amniotic membrane is an effective burn dressing material. Jpn J Surg. 1985;15:140-143. |
|
Shimazaki J, Hao-Yang Y, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmol. 1997;104:2068-2076. |
|
Shun A, Ramsey-Stewart G. Human amnion in the treatment of chronic ulceration of the legs. Med J Aust. 1983;2:279-283. |
|
Silverton JS, Trelford JD, Roussere JT, Wolfe BM, Conti S. The use of amniotic membrane in acute massive full-thickness loss of the abdominal wall from clostridial myonecrosis. Ann Plast Surg. 1979;3:558-566. |
|
Somerville PG. The possible use of amniotic membrane in chronic leg ulcers. Phlebologie. 1982;35:223-229. |
|
Subrahmanyam M. Amniotic membrane as a cover for microskin grafts. Br J Plast Surg. 1995;48:477-478. |
|
Talmi TP, Finckelstein Y, Zohar Y. Use of human amniotic membrane as a biological dressing. Eur J Plast Surg. 1990;13:160-162. |
|
Trelford JD, Trelford-Sauder M. The amnion in surgery, past and present. Am J Obstet Gynecol. 1979;134;833-845. |
|
Trelford-Sauder M, Dawe EJ, Trelford JD. Use of allograft amniotic membrane for control of intra-abdominal adhesions. J Med. 1978;9:273-284. |
|
Tseng SCG, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431-441. |
|
Tsubota K, Satake Y, Ohyama M, et al. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol. 1996;122:38-52. |
|
Tsubota K, Satake Y, Kaido M, et al. Treatment of severe ocular surface disorders with corneal epithelial stem-cell transplantation. N Eng J Med 1999;340:1697-1703. |
|
Waikakul S, Chunmniprasas K, Setasubun S, Vajaradul Y. Application of freeze-dried amniotic membrane: a control trial at the donor site of split thickness skin grafting. Bull Hosp Jt Dis Ortho Inst. 1990;50:27-34. |
|
Wang M, Gray T, Prabhasawat P, et al. Corneal haze is reduced by amniotic membrane matrix in excimer laser photoablation in rabbits. Invest Ophthalmol Vis Sci. 1997;38:S405. |
|
Welch C, Baum J. Tarsorrhaphy for corneal disease in patients with rheumatoid arthiritis. Ophthalmic Surg. 1988;19:31-32. |
|
Yokomari K, Ohkubra M, Kitano Y, et al. Advantages and pitfalls of amnion inversion repair for the treatment of large unruptured omphalocele: results of 22 cases. J Pediatr Surg. 1992;27:882-884. |
|
Young RL, Cota J, Sund G, Mason BA, Wheeler JM. The use of an amniotic membrane graft to prevent postoperative adhesions. Fertil Steril. 1991;55:624-628. |
|
Zohar Y, Talmi YP, Finkelstein Y, Shvili Y, Sadov R, Laurian N. Use of human amniotic membrane in otolaryngologic practice. Laryngoscope. 1987;97:978-80. |
Table 1 Criteria for success, partial success and failure in each category
Indication for surgery
Success
Partial success
Failure
Limbal stem cell deficiency Relief photophobia, pain,irritation, and inflammation,Decrease keratinization, Release symblepharon Relief photophobia, pain, and irritation, Decrease inflammation and keratinization Some fibrovascular tissue or slight recurrent symblepharon
Persistence of pain, irritation, and inflammation, Recurrence of keratinization and symblepharon PED, dellen, descemetocele Healed epithelial defect Incomplete healing None healing Bullous keratopathy Relief pain and irritation Transient relief of pain and irritation Persistence of pain and irritation Band keratopathy Relief pain, irritation, Delayed calcium deposit Transient relief pain, irritation, Recurrence of calcium deposit Persistence of pain, irritation, Recurrence of calcium deposit Preparing for prosthesis Wearing prosthesis without pain Decreased pain Persistence of pain, unable to wear prosthesis Corneal ulcer Healed ulcer Incomplete healing None healing Acute burn Healed epithelial defect Incomplete healing None healing
Table2 Results of corneal surface reconstruction in each indication
Results Eyes(%)
Success
Partial success
Failure
Limbal deficiency 21/27 12.1(3-29)
18 (66.7)
7 (25.9)
2 (7.4)
AMT c LT 12*/12 15.4(3-29)
11 (91.7)
1 (8.3)
0
AMT s LT 11*/15 8.5(3-17)
7 (46.7)
6 (40.0)
2 (13.3)
Bullous Keratopathy 26/26 10.0(3-24)
24 (92.3)
2(7.9)
0
PED, Dellen, Descemetocele 17/17 9.8(4-19)
17(100)
0
0
Band Keratopathy 11/11 12.1(3-27)
9 (81.8)
2 (18.2)
0
Prosthesis 1/1 3(3)
1 (100)
0
0
Corneal ulcer 1/1 5(5)
1 (100)
0
0
Acute chemical burn 1/1 4(4)
0
0
1 (100)
Note PED = persistent epithelial defect, AMT = amniotic membrane transplantation, LT = limbal transplantation
Two patients had amniotic membrane transplantation combined with limbal transplantation in one eye and amniotic membrane transplantation alone in another eye.
Definition of success, partial success, and failure is given in Table 1
Table 3: Details of surgeries and outcomes
Note In the complication, the immediate complications does not mention time in the table
AMG=amniotic membrane graft, AMT= amniotic membrane transplantation, ED=epithelial defect, EDTA= Ethylene diamine tetrachloroacetic acid LT= limbal transplantation, PED=persistent epithelial defect, PKP= penetrating keratoplasty, PO=punctal occlusion.
Table 3 Details of surgeries and outcomes
| Indications for the treatment | AMT area (extent) | Surgeries with AMT | ED healing time (weeks) | Vision change (eyes) | Complications(eyes) |
| PED, Dellen, Descemetocele | Cornea 5-9 mm | - | 2.0 | Same
15 Improve (Fc to 20/100) 1 Worse :2/0200 to HM 1 (due to cataract and uveitis) |
No |
| Limbal deficiency | Whole cornea and conjunctiva extend to 5-7 mm from the limbus | 2.8 | |||
| AMG c LT | PKP 3 Tarsorrhaphy 3 PO 5 |
2.1 | Same
2 Improve: Fc to > 5/200 1 > 20/200 2 > 20/70 7 |
Corneal Ulcer 1
(3 months) Corneal perforation 1 (at 9 months) |
|
| AMG s LT | PO
14 Tarsorrhaphy 7 Lid correction 2 |
3.7 | Same
9 Improve < 2 lines 6 |
Conjunctivitis
1 Lid abscess 1 Entropion 1 AMG detached 1 Corneal perforate 2 (at 2, 3 mo) |
|
| Bullous Keratopathy | Cornea 9 mm | - | 2.1 | same | Corneal ulcer at
1 (2 mo) AMG detached |
| Band Keratopathy | Cornea 9 mm | EDTA | 1.6 | Same | No |
| Prosthesis | Cornea 9 mm | - | 1 | same | No |
| Corneal ulcer | Cornea 4 mm | Superficial keratectomy | 1 | Improve > 2 lines 1 | No |
| Acute chemical burn | Cornea 9 mm | - | > 12 | Same | ED not healed |
| Pterygium | Conjunctiva 8-10 mm | - | 1.1 | Same Improve |
Dellen 2 eyes |
FIGURE LEGENDS
Preoperatively (left panel) and postoperative (right panel) appearance of corneal surface diseases, case 1 through 4. Preoperative appearance of Steven Johnson’s syndrome (Case 1) showed marked inflammation of the left eye (a). Postoperative AMT covered the whole cornea and bulbar conjunctiva 3 months in the left eye (b) resulting in a quiescent perilimbal conjunctiva. Case 2 limbal stem cell deficiency from HSV in the right eye, preoperatively appearance (c) showed conjunctiva marked injected and vascularization 3600 around the cornea. Post operatively 12 months of amniotic membrane transplantation (AMT) and 9 months of limbal transplantation (d) resulted in quiescent perilimbal area (AMT area) and clear cornea. Case 3 had pseudophakic bullous keratopathy (e) in the right eye. Post operatively AMT 1 month covered the cornea (f), the membrane was very transparent and smooth. Preoperative appearance of case 4, persistent epithelial defect and thinning of the corneal stroma 5 mm at the central cornea (arrow)(g). Postoperatively epithelial defect was healed completely and stromal bed thickening at 3 weeks post-op without fluorescein staining (h), amniotic membrane partially dissolved but still attached at the lesion (arrow)


Chemical Burn Limbal allograft transplantation

SJS limbal deficiency Limbal allograft transplantation
| Amniotic membrane transplantation for PED | Amniotic membrane transplantation for Bullous keratopathy |


| Amniotic membrane transplantation for conjunctival mass | Amniotic membrane transplantation for lesion |