![]()

WUTTIPONG TIRAKOTAI
ISBN 974-644-834-9, COPYRIGHT OF MAHIDOL UNIVERSITY
![]()
ACKNOWLEDGEMENT
LIST OF ABBREVIATIONS
CHAPTERS
I. INTRODUCTION
II. LITERATURE REVIEW
Chronicle of bone graft for cranioplasty |
|
Sterilization of tissue graft |
|
Bone graft incorporation |
|
Bone scintigraphy |
III. MATERIAL AND METHOD
Material |
|
Method |
IV. RESULTS
Clinical course |
|
Histology of bone graft |
|
Culture of bone graft |
|
Radiographic and bone scintigraphy results |
V. DISCUSSION
REFFERENCES
APPENDIX
BIOGRAPHY
![]()
I would like to express my deep gratitude to Professor P. Watanapa for his guidance, understanding and valuable discussion throughout the period of study for this thesis. I am deeply grateful to Assistant Professor C. Tandhavadhana who has continuously and willingly given me innovative ideas, motivation and encouragement.
I wish to thank Professor Y. Vajaradul for his advice on tissue graft processing technique and for permitting the use of Bangkok Biomaterial center service. Great appreciation is also extended to Dr. S Benjarassamerote, Dr. P. Chiewvit , Dr. S. Chiewvit for their kind help and valuable guidance in interpreting the investigation results. I am grateful to Prof. William W. Tomford, for his valuable comments and discussions as a guest observer during the thesis defence.
I am indebted to the Bangkok Biomaterial Center, Mahidol university, Mr. Reyaz A. Qayami and the other staffs for processing all bone grafts for the patients, and to the Division of Nuclear Medicine Siriraj Hospital, for granting me the permission to use the bone scintigraphy as an investigation for viability of bone graft.
I thank the neurosurgical residents for collecting grafts, I thank Ms. B. Choksawat for searching patent’s files, documents, I also thank Ms. S. Klinchan for arranging the schedule for bone scan test.
I am grateful to Assist Professor V. Chanyavanich, Dr. M. Luxsuwong,
Dr. S. Nunta-aree and other staffs in Division of Neurosurgery, Department of Surgery, Siriraj Hospital for giving me the opportunity to persue this study and I wish to thank my brother for his generous support.
Above all, I wholeheartedly acknowledge my parents Miss W. Tandhavadhana and Dr. S. Lapanich for their everlasting love and kind support.
![]()
BBC Bangkok Biomaterial Center
0C Degree Celsius
60
CO Cobalt – 60HIV Human Immunodeficiency Virus
kGy Kilo Gray
SAL Sterility Assurance Level
TCID50 /ml Tissue Culture Infective Dose per ml
99m
Tc MDP Technetium – 99m Methylene diphosphonate
CHAPTER I
![]()
One of the oldest surgical interventions is trephination of the skull. Skulls taken from the graveyards of Paracas and Parachamac in Peru indicate that this surgical procedure was practiced in the prehistory Inca Culture of 3,000 BC. As long as there have been skull defects, there have been attempts to cover them. Gold and silver pieces corresponding to the size of the holes in trephined skulls were found in these graveyards adjacent to the skulls1.
In Neuro-surgical practice, it is sometime necessary to remove a bone flap to allow swelling of the brain (external decompression) after severe head traumas, intracranial vascular events or increased intracranial pressure in brain tumor cases. The resulting skull defect is usually repaired by a cranioplasty operation several months after the flap was removed when the patient’s condition is suitable.
Large skull defects secondary to craniectomy make an area of brain more susceptible to direct trauma. Although direct brain injury following craniectomy is uncommon clinically, it remains a potential problem. The major purpose of performing a cranioplasty is to protect the brain, avoiding mechanical damages in case of trauma, and to provide a good cosmetic appearance of the head contour. Although controversial, many stress the value of cranioplasty in-patients with epilepsy following craniectomy, hemispheric collapse and syndrome of the trephined 1,2.
A variety of substitutes have been used to cover skull defects. During the last few decades, non-osseous materials (Methylmethacrylate, Ceramic) have seemingly gained popularity over the patient’s own bones. Autogenous bone has been less often preserved for delayed reimplantation. But there are evidences that cranioplasty by using the patient’s own bone flap yields good cosmetic results with a short operation time, as no modeling is required prior to the replacement3,4. Early bone conservation methods involved storage in alcohol or antiseptic solutions or sterilization with heat (autoclaving) resulted in a high rate of bone resorption. These methods destroy the osteogenic potential of the bone flap and destroy bone protein, increasing the tendency for infections and absorption5,6,7.
Deep freezing of bone is a more successful conservation method and there are clinical observations and experimental data indicates that radiation-sterilized deep frozen bone allografts are better remodeled, they also posses better Osteo-inductive and mechanical properties5,8. So we decided to introduce this new preservation and sterilization method to the cranial bone grafts, removed from the patient.
The aim of this study is to determine the use of preserved autogenous bone flaps in 15 patients, which were removed during the initial surgery and preserved at -70o C and irradiated with 25 kGy Gamma ray for cranioplasty.
CHAPTER II
![]()
“Chronicle of Bone Graft for Cranioplasty”
Physician began to experiment with bone for cranioplasty in the 19th and early 20th centuries. Macewen, in 1888, reported that re-implantation of antiseptic bone fragments could be used for cranioplasty. In 1889, Seydel transplanted an autograft of tibia with attached periosteum into a skull defect. The use of scapular bone was advocated by Mac-Lennan in 1920 and split thickness rib grafts were suggested by Fagarasanu in 19371.
The use of frozen cranial bone flaps for cranioplasty has been reported over the last 40 years5,7,9. Deep freezing of bone is a more successful conservation method. With strict maintenance at a constant temperature of -23oC to prevent bacterial overgrowth, bone can be stored successfully for many months5,10.
It is important to keep in mind that if the bone graft is exposed to air for longer than 30 minutes or is subjected to temperatures in excess of 42oC, cell viability decreases significantly. Also, physiological saline is toxic to the bone graft if the graft is exposed to it for a long time, The use of antibiotic such as bacitracin and neomycin should be avoided because these are cellucidal and significantly decrease osteogeneic capacity11.
Early bone conservation methods that involved storage in alcohol and mercury or sterilization with heat resulted in an unacceptable high rate of bone absorption5,6,7,12. After World War-I there are papers reporting that skull flap was preserved under the abdominal wall. This method is advantageous because it does not require use of a deep-freezing storage unit and is inexpensive13. However, conservation of bone under the abdominal wall requires two additional operations and produces a large abdominal scar; furthermore, the bone may eventually reabsorb. Additionally, in large de-compressive craniectomies, the bone pieces themselves may produce an unpleasant abdominal pressure sensation for the patient. Currently, this method of preserving bone is not widely used11.
“Sterilization of Tissue Graft”
In the mid 1950s, ionizing radiation was introduced for the sterilization of bone grafts14, and it is routinely uses in many tissue banks in the world. In BBC, radiation sterilization has been used since 1984.
The risk of infectious disease transmission with tissue allografts is a major concern in tissue banking practice. Microorganisms can be introduced into the grafts during tissue collection, processing and storage, but even if all these procedures are done under aseptic techniques, the chance of bacterial or viral disease transmission from the donor origin cannot be excluded. Therefore, in order to minimize the risk of disease transmission, several steps has been undertaken by tissue banks, including careful donor-screening, proper tissue processing and sterilization of tissue graft.
Bioburden and sterility of tissue allografts
Sterilization is the act or process, physical or chemical that destroys or eliminates all forms of life, especially microorganisms15. The definition is complicated by the fact that viruses are not ‘alive’, and prions are even more difficult to fit into definition. Therefore, it is advisable to use the term ‘inactivation’ in this respect. Any sterilization procedure should inactivate the types and numbers (density) of microorganisms present on the item with a defined level of sterility assurance.
The kinetics of microbial inactivation is of an exponential nature, which means that there is always a probability that some microorganisms may survive regardless of the extent of treatment applied (Fig. 1). This exponential decline is usually converted to a linear relationship by taking log10 initial contamination level (bioburden) (Fig. 2). From log10 bioburden the D10-value (D-decimal reduction) can be calculated. For many sterilization procedures this is constant, i.e. one unit dose (D10-value) will reduce the bioburden from 104 to 103, the second unit from 103 to 102, etc. By knowing the D10-value for a chosen sterilization procedure against the most resistant microorganism in various kinds of specimens, one can calculate how many D10-value are needed to reduce any expected bioburden to zero, and then below zero to give the sterility assurance level16.
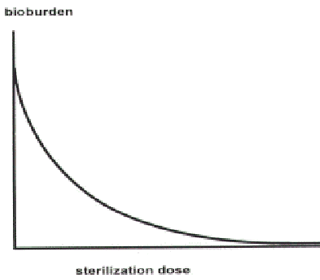
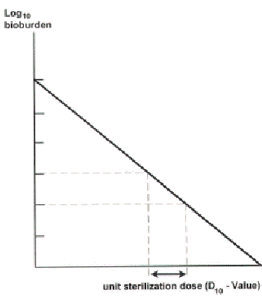
Fig. 1 Exponential decrease of bioburden as a function of radiation dose |
Fig. 2 Estimation of unit a functional sterilization dose (D10 – Value) from log10 bioburden – dose relationship |
The sterilization efficacy of ionizing radiation lies in its good penetrability inside the matter and its high killing efficiency of microorganisms. The damaging process to microorganisms maybe caused directly by ionizing radiation, or indirectly by the action of active entities, presumably hydroxyl radicals produced by ionizing radiation in water, if present in the specimen. The last mechanism is a dominating one. The specificity of ionizing radiation are its very good penetration ability and effectiveness in the inactivation of microorganisms in the bulk of any material without associated problems of heat exchange, pressure differences or hindrances by diffusion barriers17.
The radiation resistance of microorganisms is genetically determined. Usually, spores are more radiation-resistant than vegetative forms of bacteria, the most resistant fungi may be as resistant as bacterial spores, while viruses are in general, more resistant than bacteria17. Prions are extremely resistant to most of chemical and physical sterilizing agents, including ionizing radiation. In sterilization studies, it is usual to adopt a worst-case scenario, i.e. to choose the microorganisms most resistant to the applied sterilization procedure and at the highest density likely18. There is no data on the sensitivity of hepatitis B virus (HBV) to ionizing radiation; however, this virus is known to be resistant to heat sterilization.
From kinetic studies on microbial inactivation, the concept of ‘sterility assurance level’ – SAL is derived. This value is expressed as a negative power to the base of ten. For example, SAL of 10-6 would assure less than one chance in million that a contaminant would survive on or inside the object following a given sterilization process. For radiation-sterilized medical products and tissue allografts a safety margin is recommended. A sterility assurance level (SAL) of 10-6 is generally accepted19.
Most of the studies were done on the inactivation of HIV viruses, particularly in blood plasma. The dose required to eliminate all active HIV-1 from the particular tissues depends on the amount of virus originally present (initial contamination). It has been postulated that the dose of irradiation (the D10-value) needed to reduce the viral load by 1 log10 is 4 kGy20 or even 5.6 kGy 21. The level of infectious HIV in plasma is usually evaluated by titration tissue culture infective dose/ml (TCID50/ml). Talking into consideration the sterility assurance level (SAL) of 10-6, assuming HIV bioburden to be about 103 TCID50/ml for the state of acute infection22 and a D10 value of 4 kGy, the reduction of (6+3) log10 units, or a dose of 36 kGy, would be required20. On the other hand, if the D10 value is 5.6 kGy, then a dose > 50 kGy would be needed to inactivate HIV21. These results are in agreement with data published by Fideler23 who, using polymerase chain reaction (PCR), found that in order to inactivate HIV in fresh frozen bone-patellar ligament bone grafts, a dose in the range of 30-40 kGy is required.
It should be kept in mind, however, that high doses of ionizing radiation (above 40-50 kGy) may evoke numerous physical and chemical changes in tissue allografts (e.g. induction of free radicals;) which, in turn, may decrease the mechanical properties, particularly of bone24, and may diminish the biological quality, e.g. osteoinductive capacity of bone allografts. Numerous studies have demonstrated the therapeutic effects of human bone allografts irradiated usually with a dose of 25 kGy16.
Taking into consideration the deleterious effect of high doses of ionizing radiation to tissue allografts, an alternate strategy should be considered to minimize the risk of bacterial and viral infectious disease transmission of a donor origin, i.e. careful screening and selection of potential tissue donors. The need to screen for the donor was omitted in this study because the bones used on the patients were their own (autografts).
The sterilization by heat treatment (e.g. autoclaving, boiling) impairs the mechanical properties of grafts25, destroys the osteoinductive capacity of bone and reduces bone graft incorporation5,6,7. And there are many clinical observations and experimental data indicating that radiation-sterilized deep-frozen bone allografts are remodeled. They also posses better osteoinductive and mechanical properties5,8.
“Bone Graft Incorporation”
Neurosurgeons commonly see resorption of various degree affecting skull plates exteriorized during craniotomies and returned at the end of the operation (fresh autograft). With removal of its blood supply and the predictable death of its cells, the craniectomy autogenous bone flap, when reimplanted back to the cranial defect, predominantly serve as an anatomical template or passive scaffolding that must be remodeled26.
Remodeling is a complex process of resorption follow by accretion27. Necrosis of the bone is a stimulus to repair28. Resorption is a tunneling operation, productive of resorption cavities. This process depends upon the early vascularization of the graft. Osteoclasts do not play the central role to this process, and their presence may be passive29. This observation is in contrast to the central role reportedly played by osteoclasts in extra-cranial bone resorption.
The speed and success of bone re-incorporation depends on the rate of revascularization. Vascularization is an unavoidable premise of graft incorporation30. The rate of revascularization of an autograft is considerably more rapid than that of an allograft or xenograft31. Early vascularization within a few days of implantation of the graft occurs when host vessels penetrate and widen the harversian and Volkmann canals and is followed by the formation of resorption cavities. These are filled by loose vascular fibrous or granulation tissue, on the side of which osteogenesis occur. The porosity of cortical bone is mostly marked at 4 to 6 months. This process of resorption followed by accretion (creeping substitution) continues for years. There are microscopic findings in the removed plate confirming these mechanisms of bone incorporation26.
Factors that favor graft incorporation include care in preserving all viable cells within the fresh graft and a well-vascularized recipient site for the graft. Foreign substances such as bone wax are likely to impede penetration of the graft by vessels. Boiling or autoclaving the graft denatures bone protein and impairs vascularization and resorption30,32. The mechanisms involved in bone formation by autogenous grafts are still unresolved. Basically, two concepts have evolved. One mechanism, which may be referred to as the “metaplasia theory” or osteoinduction33. This theory includes the hypothesis that bone transformation occur under the influence of osteogeneic inducing substances which diffuse from the transplant site into the host’s connective tissue34. The other is osteoconduction. This process indicates the penetration of newly formed bone directly into the old bone and requires simultaneous removal of the old bone and deposition of new bone at the site of contact27.
However there are some debates about this two concepts, such as:
There is no evidence for osteoinduction, a process whereby a matrix noncollagenous protein induces pleuripotential mesenchymal cells from overlying muscle or underlying dura to from bone in adult canine skull defects35. |
|
Takagi and Urist showed that a purified bone morphogenetic protein induces the dura mater of adult rodent to from bone36. |
|
Such a protein is not available in sufficient quantity in fresh and frozen autogenous grafts, decalcified allogenous implants and powders to induce significant amounts of new bone in the adult canine skull35 and such inductive activity of the demineralized bone matrix is questionable in bone induction of primate37. |
Conclusions drawn from these canines, monkey and human studies are as follows.
Fresh skull autografts are the template for faithful remodeling of the skull. |
|
Progressively more complex processing of autogenic skull compromises its biological qualities. |
|
Processing of autogeneic skull, including heat or chemical exposures, sterilization with ethylene oxide or high dose irradiation weakens matrix proteins and mineral bonding, leading to resorption of grafts. |
|
Autografts and allografts of the skull heal through revascularization and nonosteoclastic resorption coupled with new bone formation. |
|
Calciolysis or passive diffusion of bone mineral occurs in skull implants leading to variable resorption of the templates or necrosis of exposed mature proteins. |
|
Skull grafts can heal by osteoconductive invasion of the template from the margins of the defect38. |
“Bone Scan”
Bone scan is the most commonly performed nuclear medicine study. 99mTc Methylene disphosphonate ( 99mTc MDP) is taken up by the skeleton and emit gamma rays that can be detected by gamma camera.
Radio pharmaceutical information
Mechanism of phosphate uptake in bone.
Phosphate compounds are thought to chemisorb (to absorb by chemical bonding) to amorphous bone at kink and dislocation sites on the surface of the hydroxyapatite crystal39. Following intravenous injection of disphosphonate, the half-time (t? ) clearance from the vascular to the extravascular space is between 2 to 4 minutes. By 3 hours in normally hydration, 30-40% is bound to bone, approximately 35% of the injected dose has been excreted by the kidneys, 10-15% is in other tissue and 5-10% remains in the blood40.
The skeletal uptake of a 99mTc-labelled bone-scanning agent is dependent upon the vascular supply to the bone. In numerous instances, such as in a pagetic bone lesion, increased bone uptake is associated with increased vascularity. However, another factor perhaps as important in 99mTc uptake is the nature of the calcium phosphate (Ca-p) deposited41.
Bone scintigraphy is a useful method of determining bone graft vascularity and, hence, viability at relatively early stages following grafting when estimating vascularity is important to therapeutic decision making42. This is especially true of en bloc grafts in which the donor site blood supply is maintained intact as it is transplanted to the recipient site43.
One method of assessing the viability of bone allografts has been by repeated scintigraphy, each time using a computer – generated profile slice along the axis of the graft. As the “creeping substitution” progresses, the two peaks approach one another, meeting at or near the center upon completion of the substitution. The same procedure can be undertaken with nonvascularized autograft, since the substitution process is essentially the same. This imaging process takes several weeks, but is an effective means of following the healing process. So the bone scan was introduced to this clinical study for evaluating the vascularization of the calvarial graft44.
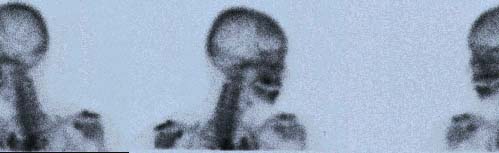
Fig. 3 Normal Bone Scan of skull
Normal appearance of head in bone scan
The most important single feature in a normal skeletal scintigram is symmetry of the midline in the saggital plane. Thus the left and right halves of the body are virtually mirror images of each other. There should be uniform uptake of tracer throughout much of the skeleton and one must be alert to any deviation from this45.
The count rate detected from the skull is comparatively low. Variations in uptake within the skull may be seen, especially in relation to the suture lines. A recent study has suggested that around 1% of patients may show this variant46. This may cause quite intense tracer uptake and may be due to subradiographic cartilaginous rests, sutural foramina or Pacchionian granulations (arachnoid extensions into the lumen of the dural sinuses). Harbert and Desai46 concluded that foci of increased uptake in the line of the sutures are likely to be benign. In older children and adolescents, difficulties may arise if it is not appreciated that there is an important suture, which can be very active, between the sphenoid and the basiocciput. This is a normal feature and should not be confused with sphenoidal sinusitis or pathology in the region of the cilvus45.
CHAPTER III
![]()
Due to high morbidity and mortality rate of the patients, selected craniectomy procedures,
coupled with little evidence of sterilization of autogenous calvarial bone graft
by irradiation, the number of cases in our study was
accepted to 15 cases.
Material
Fifteen patients who needed external decompression for reasons, which are summarized in
Table1 underwent cranioplasty procedure with preserved autogenous bone flap during
1998-2000. The age of the 15 patients ranged from 17.8 to 55.5 years (mean 32.3 years) at
the time of cranioplasty.
At the external decompression time, the removed bone flap was biopsied, then wrapped in
gauze sponge, sealed in 2 sterilized plastic bags and sent to Bangkok Biomaterial Center
(BBC). When the graft arrived BBC, it was brought out from the plastic bags and repacked
in two sterile plastic bags and vacuum sealed before irradiation with 25 kGy Gamma Ray and
stored in deep freezer at a temperature of -700 C.
On the operating day, the bone flap was brought out from the plastic bag, biopsied and
swabbed with the soaked cotton bud at inner and outer cortices from the centre to the
peripheral direction, covering 50% of the area of each side. The collecting specimen was
sent to microbiological lab within that day.
In order to study the effect of freezing and irradiation on bone morphology, bone
specimens were examined histologically. The specimens were:
Pieces of the skull fragment rongeured off from skull flap during the initial surgery as normal control. |
|
Fragments
of the bone flap which were preserved in deep freezer and irradiated. The specimens were
immersed for 24 hours in formalin, decalcified and processed for microscopic study and
interpreted by one pathologist. |
Method
The craniectomy site was reopened. After identifying
the bone edge, the bone flap was placed in the same cranial defect site and fixed with
wires. The wound was closed in layers and epidural drain was left in place for 24 hours in
all cases.
All the patients were clinically followed at least for 6 months but six of them were
observed for 10 months to 2 years time. Because of ethical reason, it is not possible to
perform sequential histological and mechanical analyses of the dynamic changes that occur
during graft incorporation. This study used indirect methods. The natural course of the
grafts were examined by plain skull film at 1st week, 1st month, 3rd month, 6th month
period, and by bone scintigraphy for evaluating the vascularity and viability of the graft
at 1st, 3rd and 6th months respectively. The results of both bone scintigraphy and plain
skull x-ray films were interpreted separately by three radiologist and one nuclear
medicine specialist doctor, without knowing the clinical result or other results beside to
the data that one was provided. From the pictures of bone scan, the interpreter divided
the bone graft areas into 4 segments, calculated the uptake of 99m Tc MDP in each area and
compared those data to the findings in each segment of the plain film (which was also
divided into 4 segments) in order to find the correlation between the findings from bone
scintigraphy and plain film appearance.
Technique of bone scan imaging
99m Tc Methylene diphosphonate (99m Tc MDP) 20 millicurie was given intravenous. In
general, imaging was preformed 3 hours after injection of the tracer. The planar image was
recorded then the Single Photon Emission Computed Tomography (SPECT) was performed by
using 2 heads gamma camera (Varicam System, Elscint) with the step and shoot technique.
Each camera rotated 1800 and each step covered 30 in 30
seconds. After that the 3 Dimension image was reconstructed. The regions of interest (ROI)
were drawn in 4 regions of bone graft (right and left of both upper and lower areas of the
graft) and at the occipital bone (as a background value). In order to measure the average
bone scan count of each ROI of graft and background.
Because we want to eliminate the factors that make the count rate of
bone graft vary from case to case, and also in one case at different time such as the
patient's hydration, the percentage of bone extraction efficiency of 99m Tc MDP, and the
excretion rate of 99m Tc MDP. The count rate that we used to calculate in the statistics
came from the value of each bone scan count divided by the value of background bone scan
count.
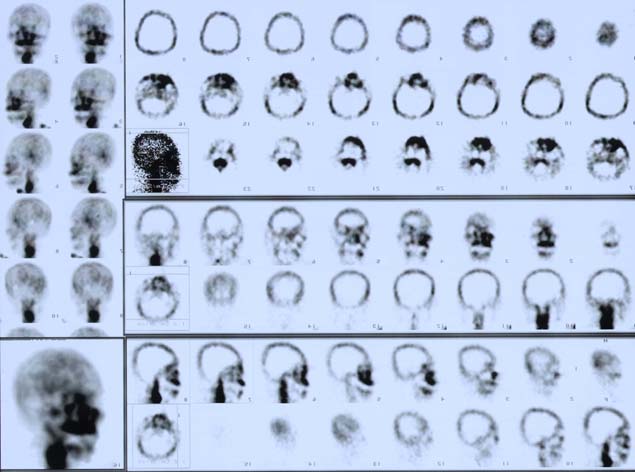
Fig. 4 Image from the SPECT and the reconstructed 3D image
Fig. 5 Vacuum packed and irradiated autograft in double sterile bags |
Fig. 6 2 Head Gamma Camera (Varicam system, Elscint) |
Flow Chart. 1 Demonstrates the process of preparing
material and study line
![]()
Plain film X-ray 1st wk, Clinically
followed up
Bone scan 1st month, 3rd month,
1st month, 3rd month,
6th month at least 6 months
6th month
![]()
![]()
![]()
3 Radiologists
Surgeon
Nuclear medicine
interpreted the result
specialist doctor
interpreted the result
Determined the similarity of each
result by using Kappa Statistics
Tab. 1 Summary cases
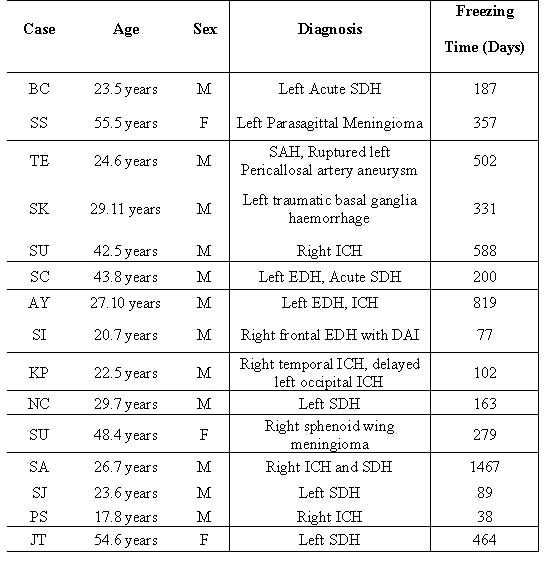
SDH = Subdural Hematoma
EDH = Epidural Hematoma
ICH = Intra-cerebral Hemorrhage
DAI = Diffused Axonal Injury
SAH = Subarachnoid Hemorrhage
CHAPTER IV
![]()
Clinical course
12 male patients and 3 female patients had undergone
cranioplasty for 16 sites. The ages at the time of cranioplasty were 17.8 - 55.5 years
(mean 32.3 years). Fourteen sites of the bone graft were successful. Major complications
included one graft resorption till loss of bone configuration. The other was removal of
bone graft because of deep infection. Two male patients had local infection. The first one
was 24.5 years old man (case TE). He had the infected wound edge with discharge at 1 month
after cranioplasty. The length of the lesion was 1.5 cm. After the debridement of the
wound edge, taking culture from the lesion and dressing, this wound became dried within 4
weeks. He was given oral antibiotics for 6 weeks. The culture result was Methicillin
Sensitive Staphylococcus Aureus (MSSA). The second case was PS. He had the erythematous,
cystic inconsistency with a 4 cm diameter mass on the scar in the center of the scalp
flap. The scar had been sutured since the time craniectomy was performed. 5 cc pus was
aspirated from this mass and sent for culture. The previous cranioplasty wound incision
was opened and explored in order to estimate the severity and extension of infection. The
pus was confined in the scalp and superficial layer of temporalis muscle. So we decided
not to remove the bone flap but debrided the debris tissue ,irrigated with aseptic
solution and gave intravenous antibiotics to the patient. After the result of the culture
was reported as Methicillin Resistant Staphylococcus Aureus (MRSA), the antibiotics were
changed to Vancomycin and it was given to the patient for 8 weeks. Both of these cases
were followed up for next 8 months without recurrence of infection.
One bone flap (case SA) had to be removed at 5th month after the cranioplasty operation
because an abscess invaded to the deep temporalis muscle. This was done because it
appeared that the infection could not be controlled. There were many osteolytic area
scattered on the bone flap. Many small capillaries from the dura mater pierced the bone
graft. The culture result was non-fermentative gram negative rod. This patient was given
oral antibiotic for 2 weeks after the removal of bone flap. One case (case KP) lost
contour of bone graft at 6th month after cranioplasty due to resorption of the occipital
bone graft but his right sided bone graft looked normal. The rest of the patients were
followed up 6 months to 2.5 years. All the reimplanted bone grafts were accepted by the
patients without postoperative complications. Three of 13 patients who had the large
fronto-temporo-parietal cranial defect had mild depression at the temporal area
corresponding to temporal fossa, which was attached by temporalis muscle.
Besides the aesthetic reason for cranioplasty, there were 6 patients who had the symptoms
and signs of atmospheric pressure effect or had the syndrome of the trephined as the
reason for cranioplasty.
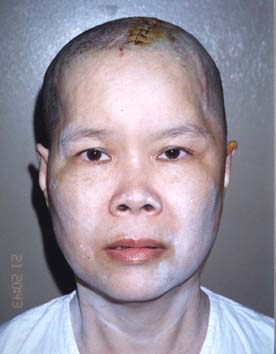
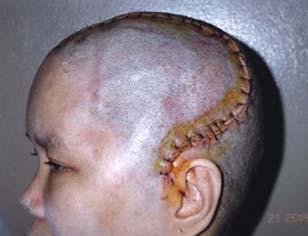
| Fig. 7 Front view of patient undergone autogenous cranioplasty | Fig. 8 Lateral view of the same patient |
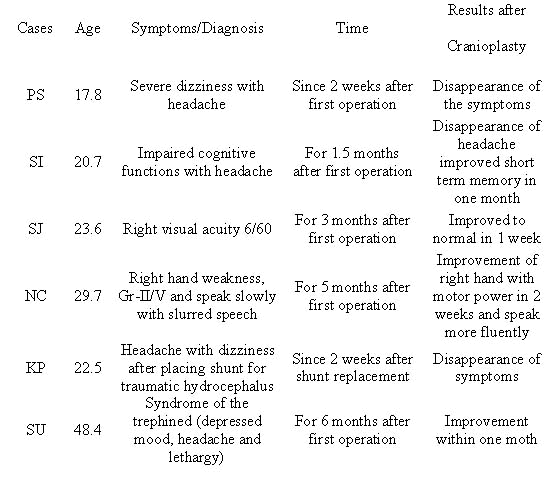
Tab. 2 Cases with symptoms and signs of the
atmospheric pressure effect or syndrome of the trephined
Histology of the bone graft
In histological findings there was no
difference between pre and post irradiated bone graft. Both showed compact lamellar bone
without osteocytes in the lacunae or some lacunae contained degenerating osteocytes.


| Fig. 9 Pre- irradiated bone graft specimen (HE x 100) | Fig. 10 Post irradiated bone graft specimen (HE x 200) |
Culture of Bone Graft
The cultures which were taken from the
bone grafts showed no growth of bacteria in the culture plates. This included both before
and after irradiation treated bone graft specimens.
Radiographic and Bone Scintigraphy results.
The roentgenograms (skull plain film)
of 11 cases showed local bone resorption at 3 months time. 10 of 11 showed increased
degree of resorption films at 6th month. Fifteen of 16 sites of the followed up films at
6th month showed bone resorption. Eight cases showed bone sclerosis at 6th month (1 case
in this group also showed bone sclerosis at the 10th month follow-up film) and 5 of 6
cases who were followed up at the 10th month showed
bone sclerosis. One case showed bone in-growth at the host graft junction from the host
side to the graft side at 10th month.
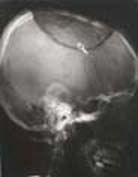
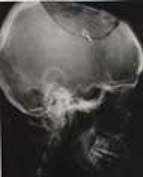
Fig. 11 X-ray at 1st month of the case showing bone in-growth at host - allograft junction |
Fig. 12 X-ray of same patient indicating bone in-growth at the host-graft junction |
The patient who had the occipital area
of bone graft failure showed osteolytic lesions at all of the 4 regions of bone graft from
the 3rd month post cranioplasty. The bone resorption progressed till the bone graft lost
its own contour at 6th month. By observing the uptake of bone scan, we conclude its
results as follows. First week after autogenous cranioplasty the bone scan revealed
decreased uptake at the bone graft in all cases. At 1 month after autogenous cranioplasty
the scan showed increased uptake in small area of 7 patients. One patient with failed bone
graft at left occipital region demonstrated significant increased uptake of about 50% in
the area of bone graft. The rest of study showed decreased uptake of bone graft at 1
month. Three months after cranioplasty, most of the cases revealed clustered areas of
increased uptake in the area of sclerosis and osteolytic lesion of plain film and
decreased uptake at the rest of graft area. The 6th month bone scan also showed area of
increased and decreased uptake in bone graft. Some areas showed high intensity of
increased uptake and some areas showed low intensity of increased uptake. One case of bone
graft failure(bone resorption)revealed increased uptake at 1 month and low intensity of
increased uptake at 3rd month and 6th month scan.
After calculating the data of bone scan count and comparing the data to the x-ray
findings, we found that the 1st month bone scan count in the group that showed osteolytic
lesion at 6th month and the group that did not show osteolytic lesion at 6th month had
significant difference of the mean value of bone scan count.(Two Sample Wilcoxon
rank-sum[Mann Whitney] test).
From Table 5 we
could find the cut off point of the bone scan count(lesion to background ratio) with its
sensitivity and specificity values. This cut off point may be used to predict the
osteolytic x-ray finding in the future. For example if we use 0.64 as the cut off point.
Even though it has the high sensitivity(93.55%) but for the specificity is only
47.06%. So the positive predictive value is (93.55/93.55+53)*100=
63.835%
And the negative predictive value is (47/47+6.45)*100=87.93%
From the Kappa statistic ( Table 6 ) we could indicate that the interpretations of the
skull films were quite the same among those 3 radiologists.
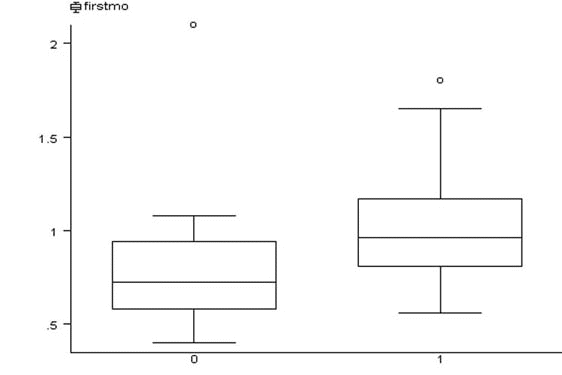
Fig. 13 Box plot: First month bone scan value (lesion to background ratio) in the
osteolytic
and non-osteolytic lesion on a 6th month x-ray film
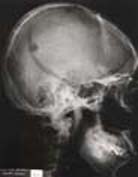
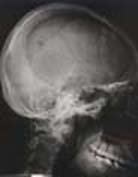
| Fig. 14 Case SJ: X-ray film at 1st month | Fig. 15 Case SJ: 6th month film showing area of osteolysis and osteosclerosis |
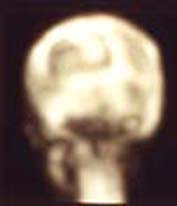
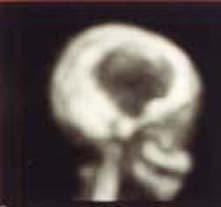
| Fig. 16 Case KP: Bone scan at 1st month showing increase uptake in 50 % of the occipital bone graft area | Fig. 17 Case KP: Bone scan at 1st month showing small area of increase uptake of right fronto-temporo-parietal bone graft |
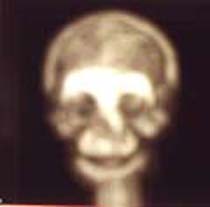
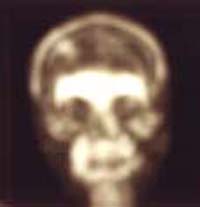
| Fig. 18 Case SI: Bone scan at 1st month showing decrease uptake of bone graft | Fig. 19 Case SI: Bone scan at 3rd month showing increase in the intensity of bone graft uptake |
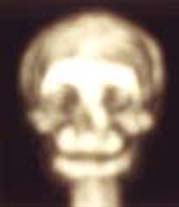
Fig. 20 Case SI: Bone scan at 6th month showing
increase in the intensity of bone graft uptake
CHAPTER V
![]()
Several kinds of artificial materials have been
used for cranioplasty by many neurosurgeons but cranial bone autograft are good materials
to repair cranial bone defects as they are radiolucent, strong enough to guarantee
adequate strength for the brain protection and provide cosmetically acceptable results.
Furthermore they are cheaper than the synthetic material and eventually incorporated into
the recipient bone, reducing the incidence of late complications4,37,47,48.
Since the original cranial bone flap gives the best cosmetic result with shorter operation
time, it should be replaced whenever the original skull bone flap is available. These
results should be considered as intermediate results since the follow-up period has been
average 1 year only.
According to subsequent reports of cranioplasty with frozen stored bone flaps, with or without autoclaving, the commonest complication is infection of the flap and surrounding tissue, with an incidence of up to 33%7,26. Among the generally used sterilization methods, such as 60Co irradiation, gaseous ethylene oxide or autoclaving, we adopted irradiation of the bone flap before surgery. This is because the irradiation can make the graft sterilized with the least side effect on collagen, bone matrix protein5,8.
The cost of processing each cranial graft at BBC was 625 Baht (US $ 15/-) where as the commercial available acrylic cranioplasty kit cost approximately 3000 Baht (US $ 75/-) and some patients may require two cranioplasty kits. The costing of the graft had been comparatively very low because the BBC procures and distributes bone autografts and allografts free of cost .Only a small percentage of the processing charge is levied on the patients.
Although irradiation may reduce some mechanical properties of the bone, but it is very safe in term of sterility24. We prefer irradiation because the purpose of cranial autograft is to provide cosmetic and structural support to the brain and not to bear any mechanical weight. It is difficult to keep out possible contamination completely. Even though the flap is absolutely aseptic during the sterilization process. The chances of contamination might happen during the time of cranioplasty surgery, one may cause the unnoticeable contamination to the surgical field or instrument. So we have to put more concentration on every step that may cause the mistake. Besides to the aseptic surgical procedure, we had to pay more attention on the following steps;
Aseptic bone graft collection |
|
Tissue graft processing |
|
| Taking the graft out of the sterilized plastic bags. |
From the data that we collected during August 1998-March 2000. Forty patients were underwent cranioplasty procedure by using synthetic material (methylmethacrylate). These cases were followed up until the end of August 2000. In three of them cranioplastic flap had to be removed within 1 year due to the infection. There were other 2 cases who had to be removed the acrylic cranial graft, but both of them had been undergone cranioplasty procedure 2,10 years ago (they were not performed the cranioplasty procedure within the period that we collected the data). When we compare the incidence of graft removal because of infection in both groups. The result is nearly the same. Three of 40 patients who used acrylic for cranial graft and 1 of 16 patients who had autogeneous bone cranioplasty had to undergo removal of the graft because of infection.(7.5% VS 6.25%).
In the long term acrylic bone is still considered as the foreign body by the host. Even though an autograft in the beginning is the dead bone but as time passes it is slowly incorporated by the host bone and converted to viable bone26. The bone graft will become more resistant to infection11. The process of bone incorporation can be observed in the x-ray findings and bone scan changes in our patients. Initially the transplanted bone autograft entered the resorption phase from the 1st until 6th month and carried on at a slow pace till complete incorporation26. Between 6th-10th month another process called osteosclerosis starts becoming dominant and carries on till complete incorporation.
In our study we had noted strong evidence demonstrating the presence of osteoinduction and osteoconduction in the cranial bone graft. This can be demonstrated by the x-ray findings which indicates osteosclerosis happen at the site of autograft which is not in contact to the host bone whereas in the x-ray film, the resorption had been noted at the site near the host-graft junction. So this osteosclerosis indicates that bone in-growth should have occurred by osteoinduction, and in the case that showed bone in-growth at the host-graft junction indicates that osteoconduction involves the bone incorporation process. We attempt to consider that bone osteoinduction and osteoconduction do happen in the graft after cranial bone graft transplantation11,49, which is different from the findings in some literatures that claim osteoconduction is the only process of new bone formation in cranial bone graft 26,38.
When exposing the cranial defect area, one should take care the previous incision in order to prevent devascularization of the scalp segment. The temporalis muscle is frequently atrophied and fixed to the dura of fronto-temporo-parietal craniectomy defects. So the temporalis muscle should be separated from the dura and secured to cranial bone graft to prevent temporal atrophy and to improve the aesthetic result11.
For timing of cranioplasty in certain cases, no consensus is possible. Cranioplasty should also be delayed in patients with latent or overt infection. The osteoplastic cranioplasty site eventually vascularized, but temporarily it must be considered a foreign body. Alloplastic material always remain foreign materials. In order to avoid the risk of infection, most neurosurgeons recommend delaying cranioplasty with a “foreign body” at least 6 months after an open (i.e. contaminated) wound or one that traverses the nasal sinuses. In clean wound cases (e.g. repair of defect after removing hemangioma of the skull) there is little argument against immediate cranioplasty50. We recommend to perform the cranioplasty as soon as possible in the case that has symptoms and signs of atmospheric pressure effect or syndrome of the trephined.
Skull roentgenography and scintigraphy with 99m Tc MDP were complementary in permitting appreciation of the stages of graft reincorporating. During the 1stor 3rd month technetium scanning after cranioplasty, areas of radioactivity corresponded to zone of mottling or bone resorption of the skull. When the osteosclerosis appeared more on x-ray films or bone graft fusing in places with the adjacent skull, the radionuclide scan remained very active. Uptake of the radioactive label was intense, whether the bone appeared mottled on roentgenograms or new bone formation and fused to the surrounding skull. So the x-ray films of the skull were more useful in interpreting the stage of re-incorporation of the autogenous graft, demonstrating early resorption (mottling) and later accretion or bone formation. Even in the autografts that were clinically satisfactory, some resorption can be detected in portions of the plates on x-ray films. First month bone scan count (lesion to background ratio) may be used to predict the 6th month x-ray finding, if it has higher specificity than this. Hence more numbers of clinical cases are needed in order to conduct the experiment, do the investigations and prove this hypothesis.
![]()
Stula D. Cranioplasty. New York: Springer; 1984.p.1-112 |
Tabaddor K, LaMorgese J. Complication of a large cranial defect. J Neurosurg 1976;44: 506-8. |
Edwards MSB, Ousterhout DK. Autogeneic skull bone grafts to reconstruct large or complex skull defects in children and adolescents. Neurosurgery 1987; 20: 273-80. |
Osawa M, Hara H, Ichinose Y, Koyama T, Kobayashi S, Sugita Y. Cranioplasty with frozen and autoclaved bone flap. Acta Neurochir (Wien) 1990; 102: 38-41. |
Abbott KH. Use of frozen cranial bone flaps for autogenous homologous grafts. J Neurosurg 1953; 10: 380-8. |
Crotti FM, Mangiagalli EP. Cranial defects repair by replacing bone flaps. J Neurosurg Sci 1979; 23: 289-94. |
Odom GL, Woodhall B, Wrenn FR. The use of refrigerated autogenous bone flaps for cranioplasty. J Neurosurg 1952; 9: 606-10. |
Dziedzic GA, Ostrowski K, Stachowicz W, Michalik J, Grzesik W. Effect of radiation sterilization on the osteoinductive properties and the rate of remodelling of bone implants preserved by lyophilization and deep-freezing. Clin Orthop 1991; 272: 30-7. |
Kreuz FP, Hyatt GW, Turner TC, Bassett AL.The preservation and clinical use of freeze -dried bone. J Bone Joint Surg 1951; 33A:863-72. |
Bush LF. The use of homologous bone grafts. J Bone Joint Surg 1947; 29: 620-8. |
Delashaw JB, Persing JA. Repair of cranial defects. In: Youmans JR, editor. Neurological surgery. 4th ed. Philadelphia: WB Sauders; 1995. p.1853-64. |
Bassett CAL. Clinical impactions of cell function in bone grafting. Clin Orthop 1972; 87: 49-59. |
Kreider GN. Repair of cranial defect by new method. JAMA 1920; 64: 1024. |
Turner TC, Bassett CAL, Pate JW, Sawyer PN, Trump JG, Wright JG. Sterilisation of preserved bone grafts by high voltage cathode irradiation. J Bone Joint Surg 1956; 38: 862-4. |
Block SS. Disinfection, Sterilisation, and Preservation. 4th ed. Philadelphia: Lea&Febiger; 1991. |
Driedzic GA. Sterilization of tissue allografts. In: Phillips GO, Versen RV, Strong DM, Nather A, editors. Advances in tissue banking vol 1.Singapore: World Scientific; 1997. p.261-83. |
Christensen EA, Kristensen H, Miller A. Radiation sterilization. In: Russell AD, Hugo WB, Ayliffe GAJ, editors. Principles and practise of disinfection, preservation and sterilization. 2nd ed. Oxford: Blackwell Science; 1992. |
Kearney JN. Sterilization of human tissue implants. British Association of Tissue Bank Newsletter 1996; 6: 2-5. |
Association For The Advancement Of Medical Instrumentation. Guidelines for gamma radiation sterilization of medical devices. Arlington: AAMI; 1984. |
Conway B, Tomford W, Mankin H, Hirsch MS, Shooley RT. Radiosensitivity of HIV-1. Potential application to sterilization of bone allografts. AIDS 1991; 5: 608-9. |
Conway B, Tomford W. Radiosentivity of human immunodeficiency virus type 1.Clinical Infectious Diseases 1992; 14: 978-9. |
Ho DD, Moudgil T, Alam M. Quantitation of human immunodeficiency virus type 1 in blood of infected persons. N Engl J Med 1989; 321: 1621-5. |
Fideler BM, Vangsness CT, Moore T, Li Z, Rasheed S. Effects of gamma irradiation on the human immunodeficiency virus. J Bone Joint Surg 1994; 76A: 1032-5. |
Rock MG. Biomechanics of allografts. In: Czitrom AA, Winkler H, editors. Orthopaedic allograft surgery. New York: Springer, Wien; 1996.p.29-37. |
Kohler P, Kreicbergs A. Incorporation of autoclaved allogenetic bone supplemented with allogenetic demineralized bone matrix. An experimental study in the rabbit.Clin Orthop 1987; 218; 247-58. |
Prolo DJ, Burres KP, McLaughlin WT, Christensen AH. Autogenous skull cranioplasty:fresh and preserved (frozen),with consideration of the cellular response. Neurosurgery 1979; 4: 18-29. |
Phemister DB. The fate of transplanted bone and regenerative power of its various constituents[abstract]. Surg Gynecol Obstet 1914; 19. |
Axhausen W. The osteogenetic phases of regeneration of bone : A historical and experimental study. J Bone Joint Surg 1956; 38A: 593-600. |
Pappas AM, Beisaw NE. Bone transplantation: Correlation of physical and histologic aspects of graft incorporation. Clin Orthop 1968; 61: 79-91. |
Kiehn CL, Cebul F, Berg M, Gutentag J, Glover DM. A study of the vascularization of experimental bone grafts by means of radioactive phosphorus and the transparent chamber. Ann Surg 1952; 136: 404-11. |
Kingma MJ, Hampe JF. The behaviour of blood vessels after experimental transplantation of bone. J Bone Joint Surg 1964; 46B: 141-50. |
Urist MR,Silverman BF, Buering K, Dubuc FL, Rosenberg JM. The bone induction principle. Clin Orthop 1967; 53: 243-83. |
Urist MR, Mclean FC. Osteogenetic potency and new bone formation by induction in transplants to the anterior chamber of the eye. J Bone Joint Surg1952; 34A: 443-70. |
DeBruyn P, Kabish W. Bone formation by fresh and frozen autogenous and homologous transplants of bone, bone marrow, and periosteum. Am J Anat 1995; 96: 375-417. |
Urist MR. Bone transplants and implants. In : Urist MR, editor. Fundamental and clinical bone physiology. Philadelphia: JB Lippincott ; 1980.p.331-68. |
Takagi K, Urist MR. The reaction of the dura to bone morphogenetic protein(BMP) in repair of skull defects. Ann Surg 1982; 196:100-9. |
Aspenberg P, Lohmander LS, Thorngren KG. Failure of bone induction by bone matrix in adult monkeys. J Bone Joint Surg 1988; 70B: 625-7. |
Prolo DJ, Oklund SA. The use of bone grafts and alloplastic materials in cranioplasty. Clin Orthop 1991; 268: 270-8. |
Jone AG, Francis MD, Davis MA. Bone scanning: Radionuclide reaction mechanism.Semin Nucl Med 1976; 6 : 3. |
Krisnamurthy GT, Huebotter RJ, Tubis M, Bladh WH. Pharmacokinetics of current skeletal seeking radiopharmaceuticals. AJR 1976; 126:239. |
Francis MD, Fogelman I. 99m Tc Diphosphonate uptake mechanism on bone. In: Fogelman I,editor. Bone scanning in clinical practice. London: Springer-Verlag; 1987. p.7-17. |
Dee P, Lambruschi PG, Heibert JM. The use of Tc-99m-MDP bone scanning in the study of vascularized bone implants: concise communication. J Nucl MED 1981; 522-5. |
Moskowitz GW, Lukash F. Evaluation of bone graft viability. Semin Nucl Med 1988; 18: 246-54. |
Berggren A, Weiland AJ, Ostrup LT. Bone scintigraphy in evaluating the viability of composite bone grafts revascularized by microvascular anastomoses, conventional autogenous bone Grafts and free nonrevascularized periosteal grafts. J Bone Joint Surg1982; 64A: 799. |
Merrick MV. The normal bone scan. In: Fogelman I, editor. Bone scanning in clinical practice. London: Springer-Verlag; 1987.p.19-25. |
Habert J, Desai R. Small calvarial bone scan foci-normal variations. J Nucl Med 1985; 26:1144-8. |
Asano Y, Ryuke Y, Hasuo M, Simosawa S. Cranioplasty using cryopreserved autogenous bone [abstract]. No To Shinkei 1993; 45(12). |
Vanaclocha V, Sapena NS, Casasola CG, DeAlava E. Cranioplasty with autogenous autoclaved calvarial bone flap in the cases of tumoral invasion. Acta Neurochir (Wien) 1997; 139:970-6. |
Vanaclocha V, Bazan A, Sapena NS, Paloma V, Idoate M. Use of frozen cranial vault bone allografts in the repair of extensive cranial bone defects. Acta Neurochir (Wien) 1997 ; 139: 653-60. |
Greenberg MS. Handbook of neurosurgery . 4th ed. Florida: Greenberg Graphics ;1997. |
![]()
Tab. 3 Values of bone scan count and the x-ray findings
| AREA | FIRSTMO |
THRMO |
SIXMO |
TENMO |
SCLERO3 |
SCLEROS |
SCLERO10 |
NORM3 |
NORM |
NORM10 |
LYTIC3 |
LYTIC |
LYTIC10 |
| SK1 | 1.15 |
1.22 |
.88 |
.. |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| SK2 | .74 |
1.55 |
1.04 |
.. |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| SK3 | .56 |
.39 |
.36 |
..! |
.00 |
.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| SK4 | .56 |
.70 |
.92 |
..! |
1.00 |
1.00 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
| SU1 | .72 |
.74 |
1.06 |
1.14 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
.00 |
.00 |
.00 |
1.00 |
| SU2 | .58 |
.68 |
.64 |
.52 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
.00 |
.00 |
.00 |
| SU3 | .58 |
.80 |
.81 |
.76 |
.00 |
.00 |
.00 |
1.00 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
| SU4 | 1.08 |
1.11 |
.96 |
.83 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
1.00 |
.00 |
.00 |
.00 |
| SC1 | 1.00 |
.85 |
.96 |
..! |
.00 |
.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| SC2 | .89 |
1.02 |
1.79 |
..! |
.00 |
1.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| SC3 | .78 |
.83 |
.75 |
..! |
1.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| SC4 | .67 |
.63 |
1.46 |
..! |
.00 |
.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| KP1 | .58 |
.70 |
.90 |
1.27 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
.00 |
.00 |
.00 |
| KP2 | .45 |
.54 |
.47 |
.62 |
.00 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
.00 |
.00 |
| KP3 | .40 |
.37 |
.34 |
.43 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
.00 |
.00 |
.00 |
| KP4 | .42 |
.38 |
.40 |
.43 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
.00 |
.00 |
.00 |
| KP5 | 1.17 |
.73 |
.73 |
.76 |
.00 |
.00 |
.00 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
| KP6 | .84 |
1.11 |
.76 |
.77 |
.00 |
.00 |
.00 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
| KP7 | .81 |
.68 |
.52 |
.52 |
.00 |
.00 |
.00 |
.00 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
| KP8 | .86 |
.82 |
.57 |
.54 |
.00 |
.00 |
.00 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
| NC1 | 1.03 |
1.81 |
1.87 |
..! |
.00 |
.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| NC2 | 1.08 |
1.12 |
1.57 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| NC3 | 1.26 |
1.49 |
.67 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| NC4 | .93 |
.90 |
1.35 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
.00 |
.00 |
..! |
| AY1 | .74 |
1.11 |
1.08 |
..! |
.00 |
.00 |
..! |
.00 |
1.00 |
..! |
1.00 |
.00 |
..! |
| AY2 | .60 |
1.04 |
1.54 |
..! |
.00 |
.00 |
..! |
.00 |
1.00 |
..! |
1.00 |
.00 |
..! |
| AY3 | .96 |
1.30 |
1.62 |
..! |
.00 |
.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| AY4 | .77 |
1.10 |
1.53 |
..! |
.00 |
.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| SU1 | 1.65 |
2.33 |
1.62 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| SU2 | 1.46 |
1.59 |
1.67 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| SU3 | 1.80 |
2.16 |
1.86 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| SU4 | 2.10 |
1.63 |
.80 |
..! |
1.00 |
1.00 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
| SA1 | .42 |
1.24 |
..! |
..! |
.00 |
..! |
..! |
.00 |
..! |
..! |
1.00 |
..! |
..! |
| SA3 | .41 |
.59 |
..! |
..! |
.00 |
..! |
..! |
.00 |
..! |
..! |
1.00 |
..! |
..! |
| SA4 | .41 |
.63 |
..! |
..! |
.00 |
..! |
..! |
1.00 |
..! |
..! |
.00 |
..! |
..! |
| SJ1 | .84 |
1.55 |
1.20 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| SJ2 | .64 |
1.34 |
1.43 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| SJ3 | .97 |
1.57 |
1.44 |
..! |
.00 |
1.00 |
..! |
1.00 |
.00 |
..! |
.00 |
.00 |
..! |
| SJ4 | .62 |
1.47 |
1.15 |
..! |
1.00 |
1.00 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
| PS1 | .82 |
1.04 |
1.60 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| PS2 | 1.23 |
1.55 |
1.60 |
..! |
.00 |
1.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| PS3 | 1.14 |
.97 |
.91 |
..! |
.00 |
.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| PS4 | .95 |
1.49 |
1.36 |
..! |
.00 |
.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| JT1 | 1.40 |
1.95 |
1.46 |
..! |
.00 |
.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| JT2 | 1.07 |
1.77 |
1.62 |
..! |
1.00 |
1.00 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
| JT3 | 1.39 |
1.58 |
1.30 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| JT4 | .80 |
.74 |
.77 |
..! |
1.00 |
1.00 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
| SS1 | ..! |
1.24 |
1.14 |
1.56 |
.00 |
.00 |
.00 |
1.00 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
| SS2 | ..! |
1.08 |
.75 |
.92 |
.00 |
.00 |
.00 |
1.00 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
| SS3 | ..! |
1.03 |
.80 |
.89 |
.00 |
.00 |
.00 |
1.00 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
| SS4 | ..! |
.76 |
.88 |
.79 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
.00 |
.00 |
.00 |
.00 |
| TE1 | ..! |
2.10 |
1.83 |
1.71 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
.00 |
.00 |
.00 |
.00 |
| TE2 | ..! |
1.90 |
1.56 |
1.51 |
.00 |
.00 |
.00 |
.00 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
| TE3 | ..! |
1.82 |
2.35 |
2.05 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
.00 |
.00 |
.00 |
.00 |
| TE4 | ..! |
1.62 |
1.23 |
1.29 |
.00 |
.00 |
1.00 |
1.00 |
1.00 |
.00 |
.00 |
.00 |
1.00 |
| SI1 | .98 |
1.35 |
1.70 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| SI2 | .96 |
1.49 |
1.76 |
..! |
.00 |
.00 |
..! |
.00 |
.00 |
..! |
1.00 |
1.00 |
..! |
| SI3 | 1.03 |
1.51 |
2.01 |
..! |
.00 |
.00 |
..! |
1.00 |
.00 |
..! |
.00 |
1.00 |
..! |
| SI4 | .94 |
1.51 |
1.68 |
..! |
.00 |
1.00 |
..! |
1.00 |
1.00 |
..! |
.00 |
.00 |
..! |
firstmo, thrmo,sixmo,tenmo : Bone Scan at 1st , 3rd , 6th and 10th months
sclero3, scleros,sclero10 : Osteolytic findings on X-ray film at 3rd , 6th and 10th months
norm3, norm, norm10 : Normal findings on X-ray film at 3rd , 6th and 10th months
lytic3, lytic, lytic10 : Osteolytic findings on X-ray film at 3rd , 6th and 10th months
area : Small segment of each bone graft
Two-sample Wilcoxon rank-sum (Mann-Whitney) test
var2 | obs rank sum expected
---------+---------------------------------
0 | 17 289.5 416.5
1 | 31 886.5 759.5
---------+---------------------------------
combined | 48 1176 1176
unadjusted variance 2151.92
adjustment for ties -1.17
----------
adjusted variance 2150.75
Ho: var1(var2==0) = var1(var2==1)
z = -2.738
Prob > |z| = 0.0062
Statistic Method employed for calculating the difference of 1st month bone scan count in the group that showed osteolytic lesion at 6th month and the group that did not show the osteolytic lesion at 6th month.
Tab. 4 Variation of cut-off point with its sensitivity, specificity and likelihood ratio
Detailed report of Sensitivity and Specificity

Fig. 20 Receiver operating characteristics curve plot between sensitivity and 1- specificity

Tab. 5 Showing reading of X-ray by each segment by radiologist. Each area has been read by three radiologists.
AREA |
ZERO |
ONE |
TWO |
THREE |
1 |
1 |
0 |
0 |
2 |
2 |
1 |
0 |
0 |
2 |
3 |
3 |
0 |
0 |
0 |
4 |
0 |
0 |
3 |
0 |
5 |
3 |
0 |
0 |
0 |
6 |
3 |
0 |
0 |
0 |
7 |
0 |
0 |
3 |
0 |
8 |
0 |
0 |
3 |
0 |
9 |
3 |
0 |
0 |
0 |
10 |
0 |
0 |
0 |
3 |
11 |
3 |
0 |
0 |
0 |
12 |
3 |
0 |
0 |
0 |
13 |
3 |
0 |
0 |
0 |
14 |
0 |
0 |
3 |
0 |
15 |
3 |
0 |
0 |
0 |
16 |
0 |
0 |
3 |
0 |
17 |
2 |
1 |
0 |
0 |
18 |
3 |
0 |
0 |
0 |
19 |
3 |
0 |
0 |
0 |
20 |
0 |
2 |
1 |
0 |
21 |
0 |
3 |
0 |
0 |
22 |
3 |
0 |
0 |
0 |
23 |
0 |
3 |
0 |
0 |
24 |
0 |
3 |
0 |
0 |
25 |
3 |
0 |
0 |
0 |
26 |
3 |
0 |
0 |
0 |
27 |
1 |
0 |
2 |
0 |
28 |
0 |
0 |
3 |
0 |
29 |
3 |
0 |
0 |
0 |
31 |
3 |
0 |
0 |
0 |
32 |
0 |
0 |
3 |
0 |
33 |
0 |
3 |
0 |
0 |
| 34 | 0 | 3 | 0 | 0 |
35 |
1 |
2 |
0 |
0 |
36 |
0 |
3 |
0 |
0 |
37 |
3 |
0 |
0 |
0 |
38 |
0 |
0 |
0 |
3 |
39 |
3 |
0 |
0 |
0 |
40 |
3 |
0 |
0 |
0 |
41 |
2 |
1 |
0 |
0 |
42 |
0 |
3 |
0 |
0 |
43 |
0 |
3 |
0 |
0 |
44 |
0 |
3 |
0 |
0 |
45 |
3 |
0 |
0 |
0 |
46 |
3 |
0 |
0 |
0 |
47 |
3 |
0 |
0 |
0 |
48 |
3 |
0 |
0 |
0 |
49 |
3 |
0 |
0 |
0 |
50 |
3 |
0 |
0 |
0 |
51 |
3 |
0 |
0 |
0 |
52 |
0 |
3 |
0 |
0 |
53 |
0 |
3 |
0 |
0 |
54 |
0 |
3 |
0 |
0 |
55 |
3 |
0 |
0 |
0 |
56 |
3 |
0 |
0 |
0 |
57 |
3 |
0 |
0 |
0 |
58 |
0 |
0 |
3 |
0 |
59 |
2 |
1 |
0 |
0 |
60 |
0 |
0 |
0 |
3 |
Zero : Osteolytic lesion of X-ray at 6th month
One : Normal X-ray at 6th month
Two : Osteosclerosis X-ray at 6th month
Three : Mix between zero and two
Area : Each segment of bone graft
Outcome | Kappa
-----------------+-------------------------------
zero | 0.8214 11.02 0.0000
one | 0.8472 11.37 0.0000
two | 0.9129 12.25 0.0000
three | 0.7676 10.30 0.0000
-----------------+-------------------------------
combined | 0.8413 17.31 0.0000
< 0.2 = poor
0.21 - 0.40 = fair
0.41 - 0.60 = moderate
0.61-0.80 = good
0.81 - 1.00 = very good
![]()
NAME Mr. Wittipong Tirakotai
DATE OF BIRTH 17th November 1971
PLACE OF BIRTH Bangkok
INSTITUTIONS ATTENDED Mahidol University, 1989 – 1995: Doctor of Medicine
Mahidol University, 1996 – 1997: Graduate Diploma in Clinical Science (Surgery)
Mahidol University, 1996-1999: Diploma Thai Board of Neurosurgery
Mahidol University, 1999 – 2000: Master of Science (Clinical Science)
POSITION AND OFFICE: 1999 – Present, Division of Neurosurgery, Department of
Neurosurgery, Faculty of Medicine, Siriraj Hospital, Mahidol University.
Position: Staff