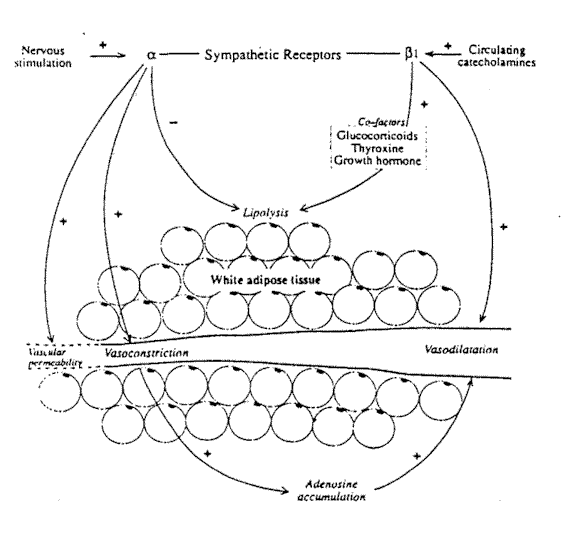
 Fig. 6-1. The metabolic and vascular effects
of sympathetic receptor stimulation in white adipose tissue.
Fig. 6-1. The metabolic and vascular effects
of sympathetic receptor stimulation in white adipose tissue.From the Department of Dermatology,
Queens Medical Center, University Hospital, Nottingham, United Kingdom.
Reprinted from Clinics in Dermatology
October-December 1989, Volume 7, Number 4, pages 62-77
Copyright 1989, Clinics in Dermatology
Courtesy of W. B. Saunders Company
Permission to display this article has been received from both the author and the publisher (details at end of article).
Strict physiological controls exist for the use and storage of adipose 'tissue so that thermoregulatory and metabolic demands for energy can be sustained. Such controls are affected by hormonal and neural mediators, often interacting both at peripheral and central sites. The role of the autonomic nervous system in regard to these functions has been widely investigated in vitro and in vivo in animals and man. The effects of classical sensory or motor components of the nervous system on adipose tissue are less clearly established. For example, pain is a feature of many disorders of fat tissue and the possible role of the autonomic nervous system versus sensory nerves in mediating pain must be considered.
The sensory nervous system has been believed to be of little importance to adipose tissue, but the recent finding of sensory nerves within adipose tissue raises issues of interest to dermatologists. In addition to a potential role in pain sensation, neuropeptides released by antidromic stimulation of sensory nerves have been shown to have an important role in cutaneous inflammation, and it is possible that they might also initiate or perpetuate inflammation within adipose tissue.
The autonomic nervous system may also play a trophic role in maintaining adipose tissue. In conditions such as scleroderma where autonomic control is deranged, there are profound changes in the subcutaneous tissues.
Factors that predispose individuals to local or generalized hypertrophy of adipose tissue are still poorly defined, but there is evidence that the autonomic nervous system may not be functioning fully in obese subjects, either as a primary or secondary phenomenon. The finding that many peptide hormones known to exist within the gastrointestinal tract also act as neurotransmitters in the central nervous system raises the possibility that these hormones may regulate appetite and satiety.
Distribution of Autonomic Nerves and Receptors in Adipose Tissue
It seems likely that major differences in adipose tissue occur from one area of the body to another depending upon the prime role of the fat in each area. The obvious differences between white adipose tissue (WAT) and brown adipose tissue (BAT) are now well recognized.
 Fig. 6-1. The metabolic and vascular effects
of sympathetic receptor stimulation in white adipose tissue.
Fig. 6-1. The metabolic and vascular effects
of sympathetic receptor stimulation in white adipose tissue.
White fat, however, may itself show wide interregional variation depending on whether it is primarily acting as support, insulation, or metabolically active tissue. Moreover, most work on the autonomic innervation of fat has been carried out in animals, and it is difficult to extrapolate this to the human situation. This is particularly true of studies on brown adipose tissue, many of which have been performed on small rodents in which the problems of thermogenesis are clearly very different from those in humans.
White Adipose Tissue
Both vascular and metabolic responses within WAT are mediated by the autonomic nervous system.1 There is little evidence for the existence of cholinergic nerve fibers in adipose tissue.2 Adrenergic fibers form a plexus lying mostly around arterioles, but with some innervation of small venules and, certainly within some fat deposits, supplying individual adipocytes.3 In addition, Fredholm has shown gap-like junctions between adipocytes that may act as a communication route between innervated and noninnervated cells.
Vascular Responses within White Adipose Tissue
Within the vessel wall are both a- and ß-adrenoreceptors. The a-adrenoceptors are located close to the sympathetic nerve terminals; that is, they are innervated terminals, while the ß receptors appear to lie closer to the vessel lumen. Thus, the ß-receptors are not directly innervated but rely on diffusion of transmitters from nearby sympathetic terminals or circulating catecholeamines. The ß receptors in adipose tissue are of the ß1 type, as opposed, for example, to muscle where ß2 receptors occur. The stimulation of. the sympathetic nerves supplying the vasculature of WAT results in several phenomena (Fig. 6-1). Vasoconstriction occurs as an autoregulatory response to a rise in vascular pressure. Sympathetic vasoconstriction has been demonstrated in humans by a variety of mechanisms including the baroreceptor response to tilting.4 Henriksen5 reviewed a series of experiments to determine whether autoregulation is mediated by local or central mechanisms. Central sympathetic blockade did not alter the response, indicating that sympathetic ganglia were not involved. Transection of somatesthetic nerves to the area also failed to inhibit autoregulation, whereas sympathectomy abolishes the vasoconstrictor reaction, indicating that it is mediated by a local sympathetic axon reflex.
In previous chapters, the importance of the characteristics of blood flow and permeability of vessels in adipose tissue have been emphasized.
Vasoconstriction occurs as a result of a-receptor activation so that blood flow in the tissue decreases proportionally to the stimulating frequency. The surface area of the capillaries within adipose tissue is reduced by sympathetic nerve stimulation. The number of capillaries perfused falls, resulting in an increased perfusion distance between parts of the tissue and the vasculature. The anticipated fall in the exchange of oxygen and metabolic products resulting from this is counterbalanced by a change in vessel permeability. Sympathetic stimulation increases vascular · permeability within adipose tissue.6 This does not occur in other tissues such as skin or skeletal muscle. This increase in permeability is inhibited by a-receptor blocking agents. It has been suggested that the endothelial cells of the vessels within adipose tissue may carry a-receptors that when stimulated, cause contraction of cells and increase the pore size between endothelial cells. Even during sympathetic induced vasoconstriction, the product of permeability and capillary surface area for solutes is increased.7 This may have important implications for the metabolic processes within the tissue.
In most circumstances, the vasoconstriction produced by sympathetic stimulation is followed by a return of blood flow towards prestimulation levels even though sympathetic activity continues. This escape phenomenon is variable in its intensity. In the adipose tissue of the omentum, the escape mechanisms are more powerful than the original vasoconstrictor response and vasodilation occurs a few minutes after vasoconstriction.1 In the subcutaneous fat depots, the autoregulatory escape is less marked.3 The vasodilator response following vasoconstriction is much reduced by ß-adrenergic blockade, indicating that activation of the ß receptors in the vessel walls is one factor in the vasodilatation. The ß receptors may be stimulated by noradrenaline diffusing across from the sympathetic nerve endings or by circulating catecholamines. Intra-arterial levels of both noradrenaline and adrenaline have been shown to be increased in humans during the vasoconstrictor response to baroreceptor activity. A further factor in the vasodilatation that follows the vasoconstrictor response to sympathetic nerve stimulation is the local accumulation of adenosine. Stimulation of the a-adrenoceptors results in a marked increase in adenosine production.8 Adenosine increases the vasodilator escape mechanism which can be partly suppressed by adenosine inhibitors.
In pathological circumstances, the sympathetic-induced vasoconstrictor reflex may be partly or completely abolished. Severe hypotension, particularly related to hemorrhagic shock, severely reduces adipose tissue function, more so than in other tissues, and may lead to permanent damage to the tissue.7 The vasoconstrictor response remains unopposed. The levels of circulating catecholamines are very high during hemorrhagic shock and seem to exert their influence via the vascular a receptors. Blocking these receptors with phenoxybenzamine results in a much higher blood flow through fat during hemorrhagic shock and allows full recovery in the tissue after restoration of blood volume.7 Patients with arterial insufficiency in the legs severe enough to cause rest pain show absence of the vasoconstrictor reflex in the ischemic areas. This may be due to the presence of vasodilator metabolites that are not cleared from the tissue.5 In a small study on two patients with venous insufficiency, Henriksen5 showed that the blood flow in the subcutaneous tissue of the legs was reduced by 50% during walking, whereas control subjects showed an increase in blood flow while walking. If this observation can be extended, it is clearly important in the understanding of the development of lower limb ulceration.
Metabolic Responses in White Adipose Tissue
The development of neural control over energy-producing processes was a great evolutionary stride enabling an organism to adapt instantly to increased energy demands.9 Once again, it is only possible to generalize about the role of the sympathetic nervous system in the metabolic processes of WAT since adipose tissue from different body sites may have specialized functions and therefore particular metabolic patterns.10 Much animal research has been performed on the epididymal fat pads that, in many respects, respond quite differently than subcutaneous or omental fat. WAT is the principal store for long-term energy demands, providing fatty acids that can be used by other tissues.11 The adipocyte cell membrane has receptors for hormones and neurotransmitters. Stimulation of ß-adrenoreceptor sites induce lipolysis, while activation of a receptors inhibits lipolytic enzymes (Fig. 6-1). These two types of receptor are coupled antagonistically to plasma membrane adenylate cyclase.12 Stimulation of the ß receptors causes activation of adenylate cyclase that increases levels of cyclic AMP (cAMP) and activates cAMP-dependent protein kinase. This in turn activates triglyceride lipase that releases fatty acids and glycerol from the triacylglycerol stored in the adipocyte.13 The lipolytic response to sympathetic stimulation is completely inhibited by ß-blocking agents.13 The stimulation of the a2-adrenoceptors on white adipocytes reduces lipolysis via the inhibition of adenylate cyclase. In addition, the other effects of a-receptor stimulation in WAT will also tend to reduce lipolysis. Vasoconstriction prevents the clearance of fatty acids while the adenosine formed during stimulation of a receptors is a potent inhibitor of lipolysis. 3
Thus it can be appreciated that the net effect of sympathetic stimulation on WAT will depend on the relative number or activity of a- and ß-adrenoreceptors. Mauriege et al.12 have studied the receptor sites in fat from different body sites of moderately obese women. The clearest finding was of a significantly higher number of a-adrenoreceptors in fat from subcutaneous sites, both in absolute terms and relative to the number of ß receptors, than in omental fat. In vitro experiments showed that the addition of adrenaline to adipocytes from femoral subcutaneous sites produced an antilipolytic action only. In contrast, the addition of adrenaline to omental adipocytes caused unopposed lipolysis. Mauriege et al.12 speculate that the functional balance between the two types of adrenoreceptor is an important factor in the control of lipolysis in different regions. Alterations of this balance could produce conditions in which the mobilization of fat is disturbed. These studies help to clarify previous work showing that fat depots with a primarily mechanical supportive role were metabolically less active. Aronovsky et al.10 showed that fat from within the orbit of cats, where it acts to cushion the optic nerve, .Was far less metabolically active than subcutaneous fat, while fat from the paw was intermediate in activity.
Stimulation of the sympathetic nervous system is not the only means by which lipolysis is increased. Circulating catecholeamines and other hormones such as ACTH, growth hormone, and glucagon also play a role.14 During times of acute stress, the calorific demands of the body are supplied by glucose. Noradrenaline, released by sympathetic stimulation, is a very potent lipolytic agent, but has little effect on glycogenolysis in the liver and muscle. Adrenaline activates not only lipolysis but also glycogenolysis, thereby releasing glucose for immediate use.9 It therefore seems likely that circulating catecholeamines are most important in the response to acute stress. Trayburn and Ashwell11 consider that in situations of moderate stress, cold, and exercise, where fatty acids are needed as fuel, the sympathetic innervation is likely to be the prime factor in increasing the rate of lipolysis. In circumstances such as starvation, where there is slow mobilization of lipids, it is likely that circulating hormones are more important.
The sympathetic nervous system is not autonomous in its actions on adipose tissue. Corticosteroids are necessary for sympathetic control to function, probably because they maintain electrolyte balance at the cellular level. Adrenalectomy abolishes the sympathetic response to cold in rats. This is restored by treatment with cortisone.9 These authors also demonstrated that thyroxine is necessary for a normal lipolytic response. In part, this is due to an interaction between thyroxine and catecholeamines, but thyroxine also appears to be necessary for the formation of the enzymes involved in lipolysis.
Extraneous agents, such as large doses of alcohol and morphine, cause increased lipolysis. This action can be blocked by chemical sympathectomy and is believed to be due to stimulation of the sympathetic system within the central nervous system.9
Thus the control of metabolism in white fat is complex and dependent on many factors, both within the adipocytes themselves and in the organism as a whole.
Brown Adipose Tissue
The prime role of this specialized form of adipose tissue is thermogenesis. Small mammals with a relatively large surface area are extremely vulnerable to cold. The metabolic processes within brown fat enable large amounts of energy to be produced as heat. The speed with which this process happens after cold challenge suggest that it is under neural control. BAT is also believed to be important in arousal from hibernation since there is a marked rise in temperature and loss of lipid from BAT during arousal.12a
The importance of BAT in humans is less clearly defined. As in other mammals, it appears to be important in the response of the neonate to cold stress.13 The role of BAT in the adult human is still not defined. Adult man would only need a tiny amount (approximately 50 g) of fully active BAT to become cold acclimatized.14 Larger amounts of less active BAT may be present and brown fat is believed to be important in thermoregulation of the newborn human.15 In the adult human, BAT seems to be found most often in the perirenal fat pads, less commonly along the neck vessels and pericardial region, and rarely in the interscapular site where it is routinely detected in small mammals.16 Increased amounts of BAT have been described around the neck in outdoor workers in a cold climate and in the perirenal fat adjacent to pheochromocytomas.17
Brown adipose tissue (BAT) has a greater blood supply and is more densely innervated than WAT. Once again, it is the sympathetic nervous system that is believed to be the most functionally important in terms of vascular and metabolic responses. The parasympathetic nervous system has not been shown to be of importance. Afferent nerves are present from brown adipose tissue and appear to be part of a reflex arc. Damage by cauterization to a small part of one interscapular brown fat pad induced hyperemia and mobilization of fat from both the injured and the contralateral fat pads.18 A dual sympathetic innervation of BAT has been demonstrated.19 As in WAT, the sympathetic nerves supplying the blood vessels of BAT are long neurons originating at the synapse with preganglionic neurons in the paravertebral chain, or in the cervical or stellate ganglia. The brown adipocytes are innervated by short sympathetic fibers arising from cell bodies in intrinsic ganglia within or close to the brown fat depot. These fibers appear to terminate on the adipose cells themselves.20
Vascular Response in Brown Adipose Tissue
Stimulation of the sympathetic nerve supply to BAT results in vasodilatation without the vasoconstrictive response seen in WAT (Fig. 6-2). The vasodilatation is mediated by ß-adrenoreceptors. It is promoted by noradrenaline and adrenaline and can be blocked by ß-receptor blocking agents.21 Himms-Hagen22 has demonstrated that these ß receptors do not belong strictly to the ßl or ß2 class, having some response patterns of both. Arch et al.23 found compounds that specifically stimulate the B-adrenoceptors in brown fat adipocytes and suggested that this tissue contains an "atypical" ß receptor. Whether this is also present in the vasculature of this tissue is not defined.
Vasodilatation in BAT may also be promoted by other substances such as glucagon, a response that is not influenced by ß-receptor blockade. Some authors have suggested that the increased blood flow caused by noradrenaline is secondary to its metabolic action with the release of vasodilator metabolites.15 It does seem that under physiological conditions in vivo, however, the increase in blood flow through BAT is dependent on an intact nervous system.24
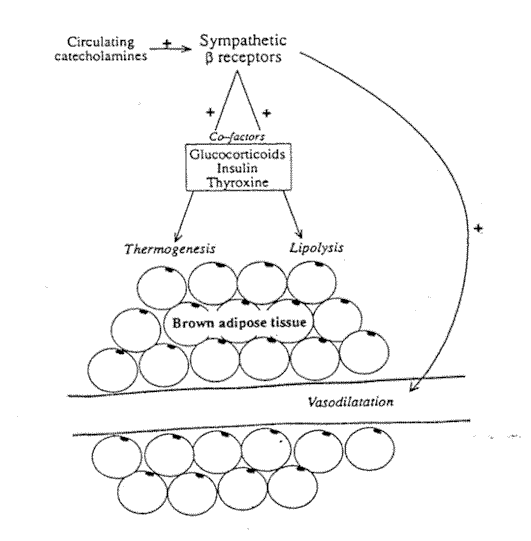 Fig. 6-2. The metabolic and vascular
effects of sympathetic receptor stimulation in brown adipose tissue.
Fig. 6-2. The metabolic and vascular
effects of sympathetic receptor stimulation in brown adipose tissue.
Metabolic Response to Sympathetic Stimulation in Brown Adipose Tissue
The specialized nature of brown adipose tissue means that it appears to respond in a more uniform way to sympathetic stimulation than WAT. The prime roles of BAT are thermogenesis and overall energy control. The sympathetic nervous system is of major, but not sole, importance in the control of these processes. As in WAT, other hormones appear to have a permissive effect on sympathetically mediated thermogenesis. Thyroxine is essential for noradrenaline to induce thermogenesis25 and insulin and glucocorticoids have also been reported to enhance the thermogenic response of BAT (Fig. 6-2).26
As described in the introduction, it is the "short" sympathetic fibers, synapsing close to the innervated tissue, that supply the brown adipocytes themselves. Activation of ß receptors on the cell surface leads not only to lipolysis as described for WAT, but also to a specific metabolic process that is not found in white adipocytes. Brown adipocytes contain many mitochondria that are able to use the fatty acids released by lipolysis for thermogenesis.27 This is an oxidative process and is normally rate limited by the amount of ADP generated during lipolysis. Brown adipocyte mitochondria have evolved a thermogenic proton conductance pathway that generates more ADP, allowing the oxidation process to continue. This pathway depends on an uncoupling protein that has been termed thermogenin. The nature of this protein and the complexities of the uncoupling mechanism have been reviewed by Cannon and Nedergaard.14 In mammals, the sympathetic nervous system is essential to maintain body temperature when the environmental temperature falls. Total chemical sympathectomy in experimental animals results in loss of shivering and nonshivering thermogenesis, and death within a few hours.9
In animals that adapt to low temperatures by hibernation, BAT accumulates before hibernation in a process seemingly related to decreasing hours of daylight.28 BAT remains the warmest part of the animal during hibernation and before arousal, becomes more metabolically active, releasing fatty acids, and helping to raise body temperature. In its role in overall energy control, brown adipose tissue serves to dispose of excess energy so that not all calories consumed will be stored. This feature can be altered so that at times when conservation of calories is required, such as pregnancy, lactation, or food restriction, thermogenesis by BAT is reduced.28 Since energy control and obesity are clearly linked, there has been much research into possible derangements of the control mechanisms of brown fat in obese animal models. These have provided fascinating insights into the possible causes and implications of obesity, but the extension of these observations to man must necessarily be cautious. The role of BAT as an energy buffer in adult humans has not been proven. Himms-Hagen28 states that the indirect evidence suggests that humans do show similar metabolic responses to cold and diet to those demonstrated in rodents and that control mechanisms may also be similar. Astrup29 reviewed the evidence for the existence and function of brown fat in adult humans. He concluded that when BAT is detected in adults, it is usually in the perirenal area, that heat increase in the interscapular area of man after noradrenaline administration was not due to the presence of BAT, and that BAT was of minor (if any) importance in thermogenesis in adult man. The possible relationship of obesity to failure of these control mechanisms is discussed later in this chapter.
Painful Disorders of Adipose Tissue
Many disorders of fatty tissue are characterized by pain. The most familiar will be the tender nodules of panniculitis, but other noninflammatory conditions may produce severe pain. These include the spectrum of Dercum's disease and other causes of painful fat around joints29 lipedema30 and painful piezogenic papules of the foot. 31 The pain of mastalgia may arise within the stroma of the breast tissue which consists largely of fat. In other fields of medicine, fat necrosis around the heart,32 and herniation of fat around the spinal column33 are well recognized causes of pain that may be difficult to diagnose. A particular feature of Dercum's disease, lip-edema, and mastalgia is that the pain often does not respond to conventional analgesics. The mechanism for pain in disorders of adipose tissue is still very ill defined, but there is evidence that the sympathetic nervous system may be able to mediate this sensation. Although the sympathetic nervous system is entirely efferent, sympathectomy relieves pain in a number of situations such as ischemic rest pain and sympathetic dystrophy. Several possible mechanisms have been Proposed to explain this phenomenon.34 It has been suggested that abnormal connections may be formed between autonomic and sensory nerves in the periphery.35 The generation of abnormal autonomic neuronal noise in the spinal column may activate pain fibers at this level.38 Others have proposed central mechanisms for this type of pain. Since the effects of sympathectomy on the denervated area are profound with increased blood flow and warmth and decreased sweating, it is possible that pain relief is secondary to these phenomena without requiring any sort of aberrant neural conduction.
Glynn, Basedow, and Walsh34 demonstrated that a regional sympathetic block of a limb with guanethidine (a competitive blocking agent of sympathetic nerve terminals); significantly reduced the pain of Raynaud's disease and sympathetic dystrophy. These authors concluded that the postganglionic sympathetic nerves were involved in pain transmission because no inhibition of the peripheral sensory nerves could be demonstrated. They also demonstrated, however, a significant increase in blood flow in the areas that might have removed pain-inducing metabolites from ischemic tissues. Other case reports present evidence that suggest that the pain and vasomotor changes are not necessarily linked. Guntheroth et al.36 described a boy with sympathetic dystrophy of one foot characterized by pain, but also by a markedly increased blood flow. All attempts at pain relief, including the use of vasoconstrictor agents were to no avail, but lumbar sympathectomy produced complete relief.
Further evidence suggesting that abnormal sympathetic activity may cause pain comes from reports of pain following sympathectomy. This has occurred after stellate ganglion block37 and lumbar sympathectomy.38 McCallurn and Glynn37 propose that increased activity in the sympathetic nerves as the effects of the anesthetic block lessen cause pain, particularly in circumstances where there was abnormal sympathetic activity before the procedure (such as sympathetic dystrophy). This type of pain is often resistant to opioid analgesics but may respond well to transcutaneous nerve stimulation. Deafferentation pain following spinal cord injury may also fail to respond to conventional analgesics and is rarely relieved by epidural local anesthesia.42 Epidural clonidine may relieve deafferentation pain where epidural morphine has failed, suggesting that the spinal noradrenergic system may be important in the transmission of pain in these patients.39
Visceral pain may also be mediated by the autonomic nervous system, and factors that induce visceral or deep somatic pain also apply to adipose tissue. Among these are sudden anoxemia, formation and accumulation of pain-producing substances, traction or compression of vessels, inflammatory states, and necrosis?
Dercums disease is a condition characterized by painful fatty deposits, usually near joints on the lower extremity. It is most common in obese, post menopausal women, but has been described in men. The original description of the disease included weakness, fatiguability, and central nervous system features such as depression, dementia, and fits.41 The latter findings appear to be very rare and some authors prefer to call the localized disease juxta-articular adiposa dolorosa.29 Histological analysis reveals apparently normal fat with no inflammatory change. The localized pads of fat are very painful, and again, conventional analgesic and anti-inflammatory treatment often fails to help. Elsman and Swezey29 suggest that the pain is caused by tension within the fat pad. There have been several reports of the efficacy of intravenous lidocaine in the treatment of this intractable condition. Skagen et al.42 described a patient who repeatedly obtained complete relief of pain for many days after lidocaine infusion. Studies of the blood flow in this case suggested that the patient had lost the vasoconstrictor reflex to limb lowering. This was believed to be due to overstimulation of the sympathetic nervous: system since lidocaine infusion produced a marked increase in blood flow to the limbs. This single observation was made in a very elderly subject, but again suggests an association between sympathetic dysfunction and pain in adipose tissue. Fat deposition within joints may also cause pain. In Hoffa's disease the infrapatellar fat pad is markedly enlarged. Premenstrual fluid retention within this fat pad is also a recognized cause of knee pain.43 Painful piezogenic papules are small herniations of encapsulated fat into the dermis (Fig. 6-3).
 FIG. 6-3. Piezogenic papules around the heel.
FIG. 6-3. Piezogenic papules around the heel.
They usually occur on the sides of the heel (pedal papules) but may also be found on the hand. Histology of the lesions shows normal fat tissue with no evidence of inflammation or necrosis." The pedal lesions may be very painful, particularly on standing. Pain is not a feature in some other structurally similar lesions, such as those that have been described on the shins. It has been suggested that here, the herniation occurs through openings in the aponeurosis that normally convey perforating venules and nerves.45 Another painful condition of adipose tissue is lipedema (See Fig. 4-10, page 45). This poorly understood disease occurs more commonly in women and may present in early adult life. Fixed, nonpitting swelling of the legs, particularly below the knee, but with sparing of the feet is evident. Swelling may be more noticeable when standing and in warm weather.29 Aching pain of the swollen areas occurs with sudden pressure on the leg producing severe pain and pinch pressure being particularly distressing.30 The fat in the affected area appears histologically normal, although it was noted to be more fluid than usual at biopsy. In most patients, there is no clinical evidence of lymphatic, venous, or arterial disease.32 Patients with lipedema tended to have somewhat higher circulating cholesterol and triglycerides than control subjects. Once again, the pain seems to be secondary to stretch and distension within the fatty tissues. It is resistant to analgesics but can be controlled by reduction of the swelling with adequate compression bandaging. Once bandaging is stopped, the swelling and pain rapidly recur.
Mastalgia may be either cyclical (maximal in the days preceding menstruation) or noncyclical when it is usually confined to one area of the breast. Cyclical mastalgia is frequently associated with fibrocystic disease of the breast and has been attributed to a hormonal effect on ductal epithelium. Neither the degree nor any particular feature of fibrocystic disease, however, has been linked with mastalgia.46 Blichert-Toft and Waat Boolser believe that the mammary stroma is the site of origin of pain in mastalgia rather than the duct epithelium. Hormonal influences on breast tissue causing edema and stretching of fat tissue may explain the cyclical nature or the pain and the response of some cases to drugs such as danazol.
Liposuction is an increasingly popular form of therapy for the removal of unwanted adipose tissue. The nature of the procedure means that the sympathetic nerve plexus within the tissue must be considerably disturbed. It is reported that pain after liposuction is usually controlled by oral analgesics. Occasionally, however, a burning pain is experienced that is resistant to usual analgesics.47 This may be due to damage to the sympathetic supply to the tissue.
The possibility that sensory innervation of adipose tissue exists has only recently been addressed and raises many interesting possibilities in regard to pain sensation, the regulation of body fat, and the role of neurogenic inflammation in inflammatory conditions of fat. There has been indirect evidence for the existence of a sensory nerve supply to adipose tissue. Neurones containing neuropeptides resembling substance P, vasoactive intestinal polypeptide, and neuropeptide Y have been demonstrated supplying the subcutaneous adipose tissue of the dog.48 This was explained by suggesting either that the neuropeptides act as cofactors in sympathetic nerves, or that they originate from sensory-nerve terminals in adjacent muscle. Evidence for a reflex arc through the spinal cord exists, however, since damage to one fat depot leads to hyperemia in the symmetrically opposite depot.24 The best evidence for the sensory innervation of fat comes from the injection of the neuroanatomical tracer "true blue." This was injected into, and carefully confined to, subcutaneous fat depots in the rat. Immuno-fluorescent histology techniques demonstrated the presence of the marker in appropriate levels of the spinal cord 7 days later.49 This study showed that sensory neural processes do originate in adipose tissue, although at present the exact site of the nerve terminals in the fat is not known.
There is now evidence that sensory nerves may be activated in the periphery through nociceptors to produce axon reflexes that result in an inflammatory response.50 Neuropeptides released from the sensory nerve endings mediate this inflammation. Substance P has been extensively studied in this role. Intradermal injection of substance P produced a similar wheal-and-flare reaction to histamine but is much more potent on a molar basis.51 It is likely that substance P exerts it inflammatory action via the release of histamine from local mast cells. Capsaicin is a substance that, when applied to skin, first releases and then depletes neuropeptides at the sensory nerve endings. Initially, inflammation follows topical capsaicin, but after repeated applications, the inflammatory reaction is abolished. Injection of histamine into areas pretreated with capsaicin still produces a wheal, but the flare response is absent, indicating that intact neuropeptide containing nerves are needed for this reaction.52 Substance P antagonists also block the flare but not the wheal response to injected substance P. Adipose tissue is known to contain mast cells (see pages 21-22).52 Although it is presently entirely speculative, it is possible that nociceptive stimuli such as trauma or severe cold could stimulate the axon reflex in sensory nerves within adipose tissue and initiate an inflammatory response. For the reasons discussed in Chapter 3, it may be particularly difficult for large molecules escaping from the vessels during such an inflammatory response to be cleared from adipose tissue, resulting in a more chronic inflammatory reaction.
Role of the Nervous System in Growth and Maintenance of Adipose Tissue
Adipose Tissue Development In Utero
In addition to genetic and maternal influences on fetal fat deposition, the brain, and particularly the pituitary gland, are important in dictating the amount and the morphology of fat deposited in the prenatal period. This in turn may influence fat deposition in the mature individual.
Much of the information on developing adipose tissue has come from the studies of fetal animals decapitated in utero at an early stage of a pregnancy that are then allowed to continue. General body growth and muscle development is not affected by decapitation, but there is an increase in body fat and growth of adipose tissue.53 Although the overall amount of fat in decapitated fetuses was greater than in controls, the subcutaneous fat layer was thinner. The adipocytes from the decapitated fetuses were morphologically similar to adult adipocytes, being larger with increased amounts of lipoprotein lipase and more surrounding collagen.54 These changes appear to be due to the unopposed influence of insulin without the normal control of fetal growth hormone. Levels of fetal growth hormone are lower in genetically obese pigs.55 In human fetuses, the period of highest growth hormone levels is also the period of lowest adipose tissue deposition.56
Control of Adipocyte Cell Size
The role of the hypothalamus and pituitary on growth of fat in adults may also be important. Surgically removed depots of fat will regrow over a period of months in experimental animals. The degree of regrowth depends on age, feeding, and hormonal status. Adipose tissue regrowth is facilitated by a fattening diet, but there is no evidence that the fat depot will regrow beyond control amounts.57 Under standard conditions of feeding, removal of one fat depot does not appear to induce compensatory growth in others. There appears to be a control mechanism on adipocyte size that is linked with satiety. Rats in whom one fat depot had been removed and a control group of sham-operated rats were fed a fattening diet. The initial increase in caloric intake was the same in both groups. Since the lipectomized rats had less total adipose tissue, their remaining adipocytes increased in size more rapidly and their hyperphagic response decreased before that of the control group. In total, the control group had overeaten more and accumulated more lipid than the lipectomized rats, but the size of adipocytes was the same in both groups.61 The feedback mechanism of adipocyte size is not known, although it has been suggested that this may be one function of the sensory nerve supply to adipose tissue.48 There has been little work on the regrowth of adipose tissue following lipectomy or liposuction in humans. Successful weight reduction is often a prerequisite for these procedures. Animal studies would suggest that the response to lipectomy may be significantly altered by preceding weight reduction. It was the one condition that invoked metabolic derangement and disfiguring compensatory hypertrophy of fat.57 Excessive enlargement of adipocytes may produce disorders of glucose and insulin metabolism.58 It may be that the amount of adipose tissue removed by lipectomy and liposuction in humans could not be sufficient to produce such effects, but studies in this area would seem to be important.
Denervation and the Accumulation of Fat
It is well recognized that regional denervation will result in accumulation of fat within the denervated area. The lipid content of the interscapular fat of mice is increased by cutting the nerve supply.9 This is presumably because sympathetic nerve induced lipolysis no longer occurs, and because the feedback mechanisms on fat cell and fat depot size are lost.
Trophic Influence of the Sympathetic Nervous System
The sympathetic nervous system also appears to exert a trophic effect on adipose tissue. It has long been recognized that lesions of localized scleroderma or morphea may correspond to nerve root, peripheral nerve, or sympathetic nerve distributions. In addition, localized scleroderma may follow skull or vertebral injuries, encephalitis, or surgical trauma.59 Jablonska59 has shown the presence of damaged and regenerating nerve fibers in the sclerodermatous tissue, in adjacent clinically normal skin, and even at distant sites, but was unable at that stage to define whether these were somatic or sympathetic. Degenerate nerves were also found in other atrophic skin diseases, but in these cases did not extend into normal paralesional skin. In a series of experiments using drugs known to affect the sympathetic nervous system at various levels, Jablonska59 showed abnormal sympathetic reactivity at both central and peripheral sites. These were most marked in patients with generalized scleroderma. Other authors have subsequently confirmed sympathetic malfunction in this condition. In a series of studies, Kristensen and Henriksen60 have defined abnormalities in the control of blood flow in generalized scleroderma. Although autoregulation of blood flow was maintained in subcutaneous tissues in this condition, it was lost in the cutaneous vessels quite early in the disease. The vasoconstrictor reflex to increased venous pressure on limb lowering was lost in both cutaneous and subcutaneous tissue in generalized scleroderma, suggesting a sympathetic neuropathy.61 There was no evidence that loss of the vasoconstrictor reflex was due to decreased vascular distensibility in the sclerodermatous tissues.62 Both the autoregulatory and vasoconstrictor reflexes help to protect against the effects of raised capillary hydrostatic pressure. In scleroderma, the cutaneous and possibly Subcutaneous tissue are less able to resist edema formation that is seen at an early stage of the disease.63 Edema fluid within connective tissue is organized by acid mucopolysaccharides and, if it persists, collagen deposition and fibrosis will occur.64 This may be one mechanism for the increase in collagen and replacement of adipose tissue that characterizes sclerodermatous tissues.
The Role of the Nervous System in Obesity
Experimental Evidence of Neuroendocrine Control of Energy Balance
Since the observations that specific areas within the hypothalamus seemed to be related to appetite and feeding, the neuroendocrine mechanisms of energy balance have been extensively studied. Although the understanding of these mechanisms is far from complete, it has become clear that the central and autonomic nervous systems and the interaction between the two are of major importance. In addition, a number of circulating peptides, many of which are common to the gut and the central nervous system, seem to play a role in feeding and satiety. Once again, most data derives from animal experiments, but work is now appearing relating the findings to human obesity. The history of the recognition of the hypothalamus as an area of weight control goes back over a century.65 Hetherington and Ransom66 showed that lesions made in a localized area of the ventromedial hypothalamus resulted in obesity due to hyperphagia. Anand and Brobeck67 demonstrated that lesions in the lateral hypothalamus resulted in decreased or absent food intake.
Thus, the "dual-center hypothesis" of energy balance was established. Stimulation of the ventromedial area of the hypothalamus (VMH) mediated satiety, while stimulation of the lateral hypothalamus invoked hunger feeding. Further evidence suggests that this hypothesis is too simple. It now appears that tracts of fibers running from the paraventricular nucleus of the hypothalamus are the critical area for satiety rather than the ventromedial nucleus? It has also been shown that obesity after VMH lesions can occur without hyper-phagia in tube fed rats.69 Bilateral hypothalamic lesions in weaning rats cause an increase in body fat without increased food intake or body weight compared to controls. Catecholeamine containing tracts also run through the ventromedial and lateral hypothalamus, some containing dopamine and others noradrenaline.65 Damage to the ventral catecholeamine bundle causes predominantly nocturnal hyper-phagia. The ventromedial hypothalamus appears to be a control region for the sympathetic nervous system.70 Lesions within the VMH result in a reduction in free fatty acid release in response to stress or cold. A similar reduction of fat mobilization from fat depots occurs after VMH lesions, as after local sympathectomy, suggesting that the sympathetic pathway to the adipose tissue runs through the VMH.71 These hypothalamic control areas are themselves sensitive to feedback mechanisms. Obesity due to VMH lesions can be prevented or reversed in a number of ways.65 Vagotomy has this effect, as does the transplantation of functioning pancreas into diabetic rats. If the diet fed to an animal with a VMH lesion is made unpalatable by the addition of quinine, obesity will not occur. Intestinal bypass also prevents or reverses VMH obesity.
Stimulation of the VMH inhibits insulin production. Since this effect can be blocked by a-adrenergic blocking agents, it appears to be mediated by the sympathetic nervous system.72 Bray and York65 hypothesize that the change in energy balance in animals after VMH lesions is a result of autonomic dysequilibrium. The sympathetic outflow is reduced and the parasympathetic outflow increased. This shift in balance results in hyperinsulinemia and altered metabolic pathways leading to obesity. During the digestion and metabolism of a meal, the autonomic nervous system provides important (but not sole) feedback control on satiety. Detection of gastric and intestinal filling is mediated via the vagus, whereas the nutritional value of the meal may be detected and regulated by local hormone pathways.73 Several hormones known to be concentrated in the upper small intestine, such as cholecystokinin, somatostatin, and bombesin have been shown to reduce food intake when they are administered parenterally.74 Glycogen levels within the liver also exert a potent feedback control.75 This feedback seems to be mediated by glucose receptors within the liver via the vagus nerve.76 These are all short-term feedback mechanisms resulting from food intake. Longer-term controls reflecting the stored calories in adipose tissue must also contribute to the regulation of energy intake. As has been discussed, adipose cell size may be one of these factors, mediated through an undefined pathway. The release of glycerol from adipose tissue is directly related to adipose tissue mass and adipocyte size, and glycerol has been suggested as one feedback control of stored energy.77 Both basal and feeding-related insulin levels rise as adipocyte size increases and fall as adipocytes shrink? The finding that insulin is found within the central nervous system (CNS) may provide another control system. Levels of CNS insulin change much more slowly than plasma insulin. In dogs, the levels of CNS insulin reflects an integral of plasma insulin over time.78 Raising the CNS insulin level in baboons by intraventricular infusion caused a significant reduction in food intake and body weight.79 The introduction of insulin antibodies into the VMH of rats produces increased food intake.80
Obesity could develop as a result of defective central or feedback controls, or because the adipose tissue itself does not respond normally to stimuli. There are several autosomal recessive inherited obesity syndromes in rodents. All of them show an increased metabolic efficiency; that is, they do not expend so many calories in thermogenesis after feeding or in the cold. After obesity has become apparent, hyperphagia and hypomobility also occur to compound the accumulation of adipose tissue. The brown adipose tissue in these rodents shows a defective response to noradrenaline and sympathetic stimulation. Animals with VMH lesions causing obesity share many of the features of genetically obese animals. One important difference is that VMH-obese animals loose the capacity for diet-induced thermogenesis while retaining normal cold-induced thermogenesis.28
Studies in Human Obesity
Considering that energy control mechanisms in the human presumably evolved at a time when considerable energy would have to be expended on the gathering of food, it is perhaps surprising that many people in the developed world manage to remain lean.73 The experimental work detailed above may offer some insights into why certain individuals become obese.
Thermogenic responses to noradrenaline have been shown to be reduced in some obese people. The resting metabolic rate of six obese women and seven lean controls was compared during intravenous infusion of noradrenaline, believed to be equivalent to that released by strenuous exercise.81 The rise in metabolic rate in the control group was over 20%, while in the obese group it was less than 10%. A group of previously obese who had dieted to near their ideal weights over a 1-2 year period still showed a response to noradrenaline similar to that of the obese group. The reduction of metabolic rate in the obese and post-obese group could not be related to decreased lipolysis since the obese group showed a higher level of plasma glycerol. The insulin/glucose ratio also rose markedly in the obese group, indicating insulin resistance.
Other groups have found that dieting may restore food-induced thermogenesis toward normal in obese subjects.82 The rise in resting oxygen consumption after a set calorie load was significantly lower in obese subjects than in controls. In the same obese subjects who had reduced weight by dieting, both resting XXX and calorie-induced oxygen showed a trend towards normal. Adipose tissue, and BAT in particular, however, are not the only tissues involved in thermogenesis in the adult human. Astrup.16 believes that the available evidence indicates that there is no thermogenic defect of BAT in obese humans and points out that skeletal muscle contributes about 50% to noradrenaline-induced thermogenesis. Peterson et al.83 studied aspects of the sympathetic and parasympathetic nervous system in a well-controlled group of obese males. There was a significant inverse correlation of plasma adrenaline and noradrenaline with percentage body fat. Increase in the pupillary latency period (involving both sympathetic and parasympathetic innervation) was significantly related to the degree of obesity. After ß blockade, the R-R interval of the cardiac cycle was inversely correlated with percentage of body fat (indicator of parasympathetic activity). There was no correlation between body fat and systolic or mean arterial blood pressure. Both sympathetic and parasympathetic activity decreased as the percentage of body fat increased. The reduced. activity of the parasympathetic nervous system differs from the predictions made from animal experiments. The authors suggest that it may be important to detect people with marked autonomic dysfunction as they may be more at risk of complications of obesity, such as hypertension and unexplained sudden death. Obesity in humans is likely to be multifactorial and it may be difficult to study a homogenous group. Dysfunction of the autonomic nervous system, whether playing a primary or secondary role, seems to be an important factor in human obesity. Better understanding of the mechanisms of energy control will help in the treatment and prevention of obesity. For example, the discovery of specific ß receptors in adipose tissue may lead to pharmacological agents that can stimulate adipose tissue lipolysis and be therapeutically useful in the treatment of obesity.23
References
| 1. | Ballard K, Rosell S. Adrenergic neurohumoral influences on circulation and lipolysis in canine omental adipose tissue. Cir Res. 1971;28:389-396. |
| 2. | Resell S. Belfrage E. Blood circulation in adipose tissue. Physiol Rev. 59 1979;1078-1104. |
| 3. | Fredholm BB. Nervous control of circulation and metabolism in white adipose tissue. In: Cryer A, Van RLR, eds: New perspectives in adipose tissue: Structure, function and development. London: Butterworths, 1985;45-64. |
| 4. | Linde B, Hjemdahl P. Effect of tilting on adipose tissuevascular resistance and sympathetic activity in humans. Am J Physiol. 1982;242:H161-HI67. |
| 5. | Henriksen O. Local sympathetic reflex mechanism in regulation of blood flow in human subcuteous adipose tissue. Acta Physiol Scand. 1977;S450:1-48. |
| 6. | Fredholm BB, Oborg G, P, Rosell S. Effects of vasoactive drugs on circulation in canine subcutaneous adipose tissue. Acta Physiol Scand. 1970;79:564-574. |
| 7. | Rosell S. Microcirculation and transport in adipose tissue. In: Renkin EM, Michel CC. eds Handbook of Physiology. The cardiovascular system Vol IV. Washington, DC: American Physiol Sec., 1964;949-967. |
| 8. | Fredholm BB, Sollevi A. The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. J Physiol. 1981;313:351-367. |
| 9. | Brodie BB. Maickel RP, Stern DN. Autonomic nervous system and adipose tissue. In: Handbook of Physiology: Adipose Tissue. Renold AE, Cahill GF. Eds. Washington, DC: American Physiol Soc., 1964;583-600. |
| 10. | Aronovsky E, Levari IL Kornblueth W, et al. Comparison of metabolic activities of orbital fat with those of other adipose tissues. Invest Ophthalmol 1963;2:59-263. |
| 11. | Trayhum P, Ashwell M. Control of white and brown adipose tissues by the autonomic nervous system. Proc Nutr Soc. 1987;46:135-142. |
| 12. | Mauriege P, Galltzky J, Berlan M, et al. Heterogenous distribution of beta and alpha-2 adrenecepter binding sites in human fat cells from various fat deposits: Functional consequences. Euro J Clin Invest. 1987;17:156-165. |
| 12a. | Nedergaard J, Cannon B. Preferential utilization of brown adipose tissue lipids during arousal from hibernation in the golden hamster. Am J Physiol. 1984;247:506-512. |
| 13. | Cannon B, Johansson BW. Nonshivering thermogenesis in the newborn. In: Baum H, Gergely J, eds. Molecular aspects of medicine, Vol 3. Oxford: Pergamon Press. 1980;119-223. |
| 14. | Cannon B, Nedergaard J. Brown adipose tissue: molecular mechanisms controlling activity and thermogenesis. In: Cryer A, Van RLR, eds: New perspectives in adipose tissue: structure, function and development. London: Butterworths, 1985;223-270. |
| 15. | Helm T, Hull D. The effect of propranalol on the calorigenic response in brown adipose tissue of now-born rabbits to catecholamines, glucagon, corticotrophin and cold exposure. J Physiol. 1966;187:271-283. |
| 16. | Astrup A. Thermogenesis in human brown adipose tissue and skeletal muscle induced by sympathomimetic stimulation. Acta Endocrinolog. 1986;112,S278:7-32. |
| 17. | Himms-Hagen J. Adrenergic receptors for metabolic responses in adipose tissue. Fed Proc. 1970;29:1388-1401. |
| 18. | Hamberger FX. Uber die innervation der Fettorgane. Z Mikroskop-Anat Forsch. 1934;36:231-266. |
| 19. | Derry DM, Schönbaum F, Steiner G. Two sympathetic nerve supplies to brown adipose tissue of the rat Can J Physiol Pharmacol. 1969;47:57-63. |
| 20. | Sidman RL, Fawcett DW. The effect of peripheral nerve section on some metabolic responses of brown adipose tissue in mice. Anat Record. 1954;118:487-507. |
| 21. | Schonbaum E, Steiner G, Sellers E A. Brown adipose tissue and norepinephrine. In: Lindberg O. ed. Brown Adipose Tissue. New York; Elsevier. 179-196. |
| 22. | Himms-Hagen J. Thermogenesis in brown adipose tissue as an energy buffer. New Engl J Med. 1984; 311(24):1549-1568. |
| 23. | Arch JRS, Ainsworth AT, Cawthorne MA, et al. Atypical ß-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 309:163-165. |
| 24. | Cottie WH. The innervation of brown adipose tissue. In: Lindberg O. ed. Brown Adipose Tissue. New York: Elsevier, 1970;155-178. |
| 25. | Rothwell N J, Stock MJ. Regulation of energy balance. Ann Rev Nutr. 1981;1',9,35-256. |
| 26. | Rothwell N J, Stock MJ. A role for insulin in the diet-induced thermogenesis in the rat. Can J Physiol Phsrmacol. 1981;58:542-848. |
| 27. | Hittelman KJ, Lindberg. Fatty acid uncoupling in brown fat mitochondria- In: Lindberg O. ed. Brown Adipose Tissue. New York; Elsevier. 19/0;245-262. |
| 28. | Himms-Hagen J. The role of brown adipose tissue thermogenesis in energy balance. In: Cryer A, Van RLR, ads. New perspectives in adipose tissue: Structure, Function and Development. London: Butterworths, 1935;199-221. |
| 29. | Elsman J, Swezwy RL, Juxta-articular adiposis dolorosa What is it? Report of 2 cases. Ann Rheum Dis 1979;38:479-482. |
| 30. | Stallworth JM, Hennigar GR, Jonsson HT, et al. The chronically swollen painful extremity. JAMA. 1974;28:1656-1659. |
| 31. | Woerdeman MI, van-Dijk E. Piezogenic papules of the feet Acta Derm Venereol (Stockh). 1972;52:411-414. |
| 32. | Behrendt DM, Scannell JG. Pericardial fat necrosis. An unusual cause of severe chest pain and thoracic "tumor." N Engl J Mad. 1968;279:473-475. |
| 33. | Faille RJ. Low back pain and lumbar fat herniation. Am J Surg. 1978;44:359-361. |
| 34. | Glynn C J, Basedow RW, Walsh JA. Pain relief following post-ganglionic sympathetic blockade with i.v. guanethidine. Br J Anassth. 1981;63:1297-1302. |
| 35. | Doupe J, Cullen CR, Chance CQ. Post-traumatic pain and the causalgic syndromes. J Neurol Neuresurg Psychiatr. 1944.7:33-48. |
| 36. | Guntherorb WG, Chakmakjian S, Brena SC et al. Postraumatic sympathetic dystrophy. Amer J Dis Child. 1971;121:511-514. |
| 37. | McCallurn MID, Glynn DJ. lntercostal neuralgia following stellate ganglion block. Anaesthesia. 1986:41:8,50-852. |
| 38. | Tracy GD, Cockett FB. Pain in the lower limb after sympathectomy. Lancet 1957;1:12-13. |
| 39. | Glynn CJ. Teddy PJ. Jamous MA, et al. Role of spinal noradrenergic system in transmission of pain in patients with spinal cord injury. Lancet 1938;2(8518) 1249-1250. |
| 40. | Procacci P, Zoppi M, Maresca M. Clinical approach to visceral sensation. Prog Brain Res. 1938;67:21-28. |
| 41. | Blomstrand R, Juhlin L, Nordensiam H, et al. Adiposis dolorosa associated with defects of lipid metabolism. Acta Derm Venereol (Stockh). 1971;51:243-250. |
| 42. | Skagen K. Petersen P, Kastrup J, et al. The regulation of subcutaneous blood flow in patient with Dercum’s disease. Acta Derm Venereol (Stockh). 1986;66:337-339. |
| 43. | Smillie IS. Lesions of the intra-patellar fat pad and related synovial membrane diseases of the knee. joint Edinburgh: Churchill-Livinstone, 1974;394-398. |
| 44. | Harman RRM, Matthews CNA. Painful piezogenic pedal papules. Br J Dermatol. 1973;90:.573-574. |
| 45. | Grob J J, Collet-Villette AM, Bonerandi JJ. Hernies piezogeniques cellulo-adipeuses de jambe. Ann Dermatol Venereol. 1987;114:1567-1569. |
| 46. | Blichert-Toft M, Watt-Boolsen S. Clinical approach to women with severe mastalgia and the therapeutic possibilities. Acta Obstet Gynecol Scand Suppl. 1984;123:185-188. |
| 47. | Illouz YG. Liposuction--The Franco-American Experience. California: Medical Aesthetics Inc. 1985;194-195. |
| 48. | Fredholm BB, Roseil S. Effects of adrenergic blocking on lipid mobilization from canine subcutaneous adipose tissue after sympathetic nerve stimulation. J Pharmacel Exp Ther. 1968;159:1-7. |
| 49. | Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol 1997.22:R942-R944. |
| 50. | Foreman JC. Neuropeptides and the pathogenesis of allergy. Allergy. 1987;42:1-11. |
| 51. | Foreman JC, Jordan CC, Oehme P, et al. Structure-activity relationships for some substance P-related peptides that cause wheel and flare reactions in human skin. J Physiol. 1963;335:449-465. |
| 52. | Slavin BG. The morphology of adipose tissue.. In: Cryer A and Van R L R ads. New perspectives in adipose tissue: structure, function and development London: Butterworths, 1965;23-43. |
| 53. | Martin R J, Kasser TR, Ramsay TG, et al. Regulation of adipose tissue development in utero. In: Cryer A, Van RLR, eds. New perspectives in adipose tissue: Structure, function and development London: Butterworths, 1985;303-317. |
| 54. | Hangman G J, Campion DR, McNamara JP, et al. Adipose tissue development in the fetal pig after decapitation. J Anim Sci. 1981;53:1634-1644. |
| 55. | Martin R J, Sheahan J, Ramsay T, et al. Hormone levels in the pre-obese fetal pig(abstract); Fed Proc. 1982;41.715. |
| 56. | Widdowson EM, Spray CM. Chemical development in utero. Arch Dis Child. 195t;26:205-214. |
| 57. | Faust IM, Kral JG. Growth of adipose tissue following lipectomy. In: Crier A, Van RLR. eds. New perspectives in adipose tissue: structure, function and development. London: Butterworths, 1985;319-332. |
| 58. | Schneider BS, Faust IM, Hemmes RB, et al. Effects of altered adipose tissue morphology on plasma insulin levels in the rat. Am J Physiol. 1981;240:E358-E362. |
| 59. | Jablonska S. The nervous system in scleroderma. In: Jablonska S. ed. Scleroderma and Pseudescleroderma (2nd edition). Warsaw: Polish, Medical Publishers, 1975;65-33. |
| 60. | Kristensen JK, Henriksen O. Local regulation of blood flow in cutaneous tissue in generalized scleroderma. J Invest Dermatol. 1978;70:260-262. |
| 61. | Henriksen O, Kristensen JK, Wadskor S. Local regulation of blood flow in subcutaneous tissue in generalized scleroderma. J Invest Dermatol. 1977;68:318-321. |
| 62. | Kristensen JK, Henriksen O. Distensibility of the vascular bed in subcutaneous tissue in generalized scleroderma. J Invest Dermatol 1978;70:156-158. |
| 63. | O'Leary PA., Montgomery H, Ragedale W E. Dermatohistopathology of various types of scleroderma. Arch Dermatol. 1957;75:78-87. |
| 64. | Asboe-Hansen G, Dyrbye MO, Moltke E, et al. Tissue oedema: a stimulus of connective tissue regeneration. J Invest Dermatol. 1.959;32:505-507. |
| 65. | Bray GA., York DA.. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev. 1979;59:.719-809. |
| 66. | Hetherington A.W, Ranson SW. Experimental hypothalamico-hypophyseal obesity in the rat. Proc Soc Exp Biol Med. 1939;41:465-466. |
| 67. | Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24:123-146. |
| 68. | Sclafani A, Berner CN. Hyperphagia and obesity produced by parasagittal and coronal hypothalamic knife cuts--Further evidence for a longitudinal feeding inhibitory pathway. J Comp Physiol 1977;91:1000-1018. |
| 69. | Han PW. Energy metabolism of tube-fed hypophysectomized rats bearing hypothalamic lesions. Am J Physiol 1968;215:1343-1350. |
| 70. | Ban T. Fiber connections in the hypothalamus and some autonomic functions: Central neural control of eating and obesity. Pharmacol Biochem Behav. 3, Suppl. 1975;1:3-13. |
| 71. | Bray GA., Nishizawa Y. Ventromedial hypothalamus modulates fat mobilization during fasting. Nature. 1978;274:900-902. |
| 72. | Woods SC, Porte D. Neural control of endocrine pancreas. Physiol Rev. 1974;54:596-619. |
| 73. | Van Itallie TB, Kissileff HR. Physiology of energy intake: an inventory control model. Am J Clin Nutr. 1985;42:914-923. |
| 74. | Woods SC, McKay D, Stein LJ, et al. Neuroendecrine regulation of food intake and body weight. Brain Res Bull 5, Suppl. 1980;4:1-5. |
| 75. | Russek M. Demonstration of the influence of an hepatic glucose sensitive mechanism on food intake. Physiol Behav. 1970;5:1207-1209. |
| 76. | Niijima A.. Afferent impulse discharges from glucoreceptors in the liver of the guinea pig. Ann NY Acad Sci. 1969;157:690-700. |
| 77. | Grinker J, Strohmayer A J, Horowitz J, et al. The effect of the metabolite glycerol on food intake and body weight in rats. Brain Res Bull 5 Suppl. 1980;4:29-35. |
| 78. | Woods SC, Porte D. Relationship between plasma and cerebrospinal fluid insulin levels of dogs. Am J Physiol. 1977;233:E331-E334. |
| 79. | Woods SC, Lotter EC, McKay LD, et al. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503-505. |
| 80. | Strubbe JH, Mein CG. Increased feeding in response to bilateral injection of insulin antibodies in the VMH. Physiol Behav. 1977;19(2):309-313. |
| 81. | Jung PT, Shetty PS, Barrand M, et al. Role of catecholeamines in hypotensive response to dieting. B J Med. 1979;1:12-13. |
| 82. | Schwarts RS, Halter JB, Bierman El. Reduced thermic effect of feeding in obesity: role of norepinephrine. Metabolism. 1983;32(2):114-117. |
| 83. | Peterson HR Rothschild M, Weinberg CR, et al. Body fat and the activity of the autonomic nervous system. N Engl J Med. 1988;318:1077-1083. |
Address for correspondence: Katharine Dalziel, MD, Consultant Dermatologist, Royal Cornwall Hospital, Treliske, Truro TR1 3LJ, Cornwall, UK
© 1989, Clinics in Dermatology. Reprinted here with the kind permission of the author, Dr. Katharine Dalziel, and the publishing house. The article was published in Clinics in Dermatology, which is published by Elsevier Science, publishing division of Harcourt Brace & Co., courtesy of W.B. Saunders Company. The actual permission and limitations statement is attached below for your information.

Return to the List of Articles
Return to the
Dercum's Disease Home Page
Last updated 18 Nov 2003
Comments about the web page format
should be sent to the Don
Please don't forget that the information provided on this site is designed to support, not replace, the relationship that exists between a patient and his or her physician.