
What is a Photoelectric Cell?
A photoelectric cell is a device
that is activated by electromagnetic energy in the form of light waves.
Photoelectric cell exists in many types, and they are used for many things. It
is a type of electric cell whose operation depends upon the extent to which it
is exposed to light. The most familiar one is when a door seem to open by itself
when we approach it, as we often see at airports. This happens because our body
blocks a beam of light and a photoelectric cell makes the door open.
Light is a form of energy. When light strikes certain chemical substances, such
as selenium and silicon, its energy causes a push on the electrons in the
substances.
Photoelectric cells exists in three basic kinds, corresponding to the three
different forms of the photoelectric effect that they employ: the
photoconductive cell, the photoemissive cell, and the photovoltaic cell.
Photoconductive Cell
The oldest photoelectric device is the photoconductive cell. In
the later 19th century it has been developed in basic form. This device is also
known as a photoresistor.
The photoconductive cells are used in many things and in many ways, for example
in turn street lights on and off automatically, as counting devices on
production lines, in various alarm systems, and in supermarkets as the sensor
that scans codes on grocery items at checkout counters. It mostly used in
photography as the light meters used to measure the intensity of illumination.
The Energy of light in modern photoconductive cell is used to free electrons
from their valence bonds in a semiconductor material. At room temperature, 21°C
(294.16K), the number of free charges in a semiconductor is relatively limited,
so the addition of light-released electrons raises its conductivity (therefore,
reduces its resistance). The resistance may change from several hundred thousand
ohms in the dark to a few hundred ohms in sunlight. To increase the dark
resistance and reduce the dark current, the conducting path is often laid down
in a zigzag manner on a ceramic wafer.
A number of substances are photoconductive. Some of which are Lead sulfide (PbS),
lead selenide (PbSe), and lead telluride (PbTe) are sensitive to infrared
radiation, whereas cadmium sulfide (CdS) has sensitivity to light in the visual
range. Changes in output current are about one milliampere (mA) per lumen.
Photoemissive Cell
The photoemissive cell, known also as phototube, first
appeared in the early 1920. These cells are familiar as the "electric
eyes" that trigger the automatic opening of doors when a person intercepts
a beam of light. These cells can also be used in a way similar to those of
photoconductive cells, in order automate and control systems. They are used in
astronomy, in the form of photo-multiplier tubes, to measure electromagnetic
radiation from celestial objects.
A photoemissive cell is constructed with a wire anode and a semi-cylindrical
cathode with an emitting surface, sealed in an evacuated or gas-filled bulb. As
cathode surfaces, monatomic layers of cesium, potassium, or rubidium are used.
Due to photons that strike the cathode, and therefore transfer their energy to
the surface electrons, some electrons can overcome the binding force and be
emitted into space. These emitted electrons are attracted to the positive anode
as a photocurrent of microampere order. For many applications, phototubes were
placed by semiconductor photodiodes.
Photovoltiac Cell
The first photovoltaic cells appeared shortly after the
photoemissive cell. They are now used in a wide range of electronic systems, an
example being modulated-light systems such as fibre optics communications
arrays. When the Sun is the source of light they are known as solar cells, and
their many applications are described more fully in that entry.
Semiconductor, of such material that light flux falling in it displaces
electrons from some of the atoms, is the sensitive element in a photovoltaic
cell. The most suitable semiconductors for photovoltaic cells are selenium and
cuprous oxide.
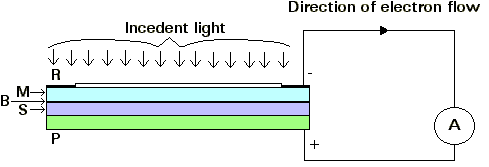
Figure 1: The arrangement of a cell having selenium as the active
material.
A steel plate P is coated with a thin layer S of Selenium
at about 200°C (473.16K) and annealed at about 80°C (353.16K) to produce the
crystalline form. This selenium layer is covered with a very thin transparent
film M of metal, and a collecting ring R of metal is sprayed around the edge of
the film. Between the selenium S and the film M, there appears to be a 'barrier
layer' B.
When the light falls on the cell, it passes through M and causes electrons to be
released from the metallic selenium. These electrons travel across B to M, from
which R collects them. A micro-ammeter A is connected between R and P. It is
found that with a suitable resistance of the external circuit between R and P,
the current through A is practically proportional to the illuminance and A can
be calibrated to read the illuminance directly in lux.





![]()