



|
Most Gram-positive bacilli encountered in clinical specimens represent normal flora which are contaminants, including members of the genera Bacillus, Lactobacillus, and Corynebacterium. Gram-positive rods which are potential pathogens are often identified by a stain other than the Gram stain (e.g., acid-fast stain for Mycobacterium and modified acid-fast stain for Nocardia). Listeria and Erysipelothrix are uncommon isolates whose identification relies on colony morphology and biochemistry, as discussed below. On a direct Gram stain of a clinical specimen, Bacillus, Lactobacillus, and Corynebacterium all have distinctive, characteristic morphologies and arrangements so that in fact the genus designation is usually apparent from the Gram stain alone, although catalase testing of colonies is also very helpful (the anaerobic clostridia are or may be indistinguishable from Bacillus on Gram stain). However, all three of these genera contain numerous species and all demonstrate wide ranges of variation in microscopic morphology. The most commonly encountered Gram-positive rods fit the following patterns:
|

After coagulase-negative staphylococci, the coryneforms (diphtheroids) are the most common skin contaminants encountered in the laboratory. These organisms are also found as indigenous flora of the nasopharynx, oropharynx, urogenital tract, and intestinal tract. They are small, highly pleomorphic Gram-positive rods, often occurring with club-shaped ends which contain intracellular polyphosphate granules (metachromatic granules when stained with methylene blue). They often fail to detach after cell division, so on a Gram stain they appear to palisade in a characteristic "Chinese letter" arrangement. They usually produce tiny to small colonies and grow rather slowly. Some strains are anaerobic; these often can be anticipated from the direct Gram stain, for their microscopic morphology resembles a collection of "spider legs". Corynebacterium spp. are strongly catalase-positive; if the catalase test is unexpectedly negative, rule out Erysipelothrix rhusiopathiae (see below). |
|
As a result of immunization, Corynebacterium diphtheriae is rarely isolated in the United States. The physician usually suspects pharyngeal diphtheria when a gray-white pseudomembrane of lymphocytes, plasma cells, cellular debris, fibrin, and bacteria is observed adhering tenaciously from the involved tissue and extending from the oropharynx to the larynx and into the trachea. Only strains of Corynebacterium diphtheriae which are lysogenic for a bacteriopage (beta phage) which carries the diphtheria exotoxin gene (the tox+ gene) are capable of producing the toxin. Diphtheria exotoxin is produced locally in the throat and subsequently is absorbed through the mucosa and is circulated to distant organs. The toxin consists of subunits A and B. Subunit B is involved in attachment of the toxin to the host cell membrane and transportation into the cell. Subunit A inhibits protein synthesis by binding to and inactivating elongation factor 2. The toxin affects both the structure and function of cardiac muscle and causes demyelination of peripheral and cranial nerves. Resulting cardiac insufficiency can be fatal. Neural paralysis is usually reversible as the myelin sheath reforms. Either toxigenic or nontoxigenic cutaneous diphtheria can occur when C. diphtheriae colonizes a break in the skin to produce a characteristic pathologic process. These lesions have frequently been associated with insect bites. The organism remains localized, but systemic effects may occur due to absorption of exotoxin into the tissues. |
|
Originally given the designation of "group JK" diphtheroids by the Centers for Disease Control (CDC), these organisms have now received the (unfortunate) species name C. jeikeium. They have emerged as a potential but uncommon cause of nosocomial infection in immunocompromised patients, where they can cause wound infections, septicemia, and endocarditis. They are particularly troubling because they tend to be susceptible to vancomycin but resistant to most antibiotics commonly used to treat Gram-positive infections. |

After coagulase-negative staphylococci and diphtheroids, members of the genus Bacillus are the third most common skin contaminant found in clinical specimens. On Gram stain they are large, wide, Gram-positive rods, often occurring singly or in pairs, which can produce endospores. They may be confused with clostridia on direct stains from specimens. Usually they are readily distinguished from lactobacilli (see below), which tend to occur as long, narrow Gram-positive rods which often chain. Also, Bacillus spp. are catalase-positive, while lactobacilli are not. Colony morphology of Bacillus spp. is highly variable, often growing as large to very large gray-white colonies that may be dry in appearance, or which may produce a contiguous mat of wet, blistery colonies. Some species are facultative anaerobes while others are obligate anaerobes. Many species are beta-hemolytic on sheep blood agar (which has no correlation with their ability to lyse human erythrocytes). There are very many species of Bacillus, only a few of which are associated with human infection. |

B. anthracis, the etiologic agent of anthrax, is a large bacillus (3 - 5 microns long) which usually occurs singly or in pairs. The ends of the cells are sharply squared off. Colonies are large, gray-white, and raised with an irregular margin, and tangled, hair-like masses of cells produce a "medusa-head" appearance. B. anthracis is a very rare human isolate in the United States. |
|
Bacillus cereus is a beta-hemolytic, Gram-positive, facultatively aerobic sporeformer. B. cereus is rarely encountered as a pathogen in human specimens. |
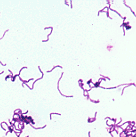
The lactobacilli are nonsporulating, Gram-positive bacilli classified in the large family Lactobacillaceae. Often they are found as long, slender Gram-positive rods in long chains. Usually they are contaminating commensals that are readily recognized from colony morphology and the fact that they are catalase-negative. However, their apparent presence in wound cultures or blood cultures suggests that Erysipelothrix rhusiopathiae should be ruled out with innoculation of a TSI slant (see below). Furthermore, Leuconostoc should be excluded in blood cultures, although Leuconostoc more closely resembles streptococci on Gram-stain. Leuconostoc is PYR-positive, catalase-negative, and resistant to vancomycin; lactobacilli are catalase-negative and PYR-variable. |
|
Listeria monocytogenes microscopically presents as a short coccobacillus, occasionally seen in short chains, sometimes encountered inside PMN's, especially in cerebrospinal fluid. The organism displays a typical "heads-over-tails" type of motility. On sheep blood agar the organism is indistinguishable from Group B Streptococcus agalactiae, for which it is often initially mistaken: a medium hazy gray colony with small zone of beta-hemolysis. However, Listeria monocytogenes is catalase-positive, while Group B Streptococcus is catalase-negative. Corynebacterium, Bacillus, and Lactobacillus are isolated much more frequently from human clinical specimens than is L. monocytogenes, although L. monocytogenes is recovered as a pathogen much more often than is B. anthracis, B. cereus, or E. rhusiopathiae, and clinical microbiologists must be very familiar with this organism and its microsocpic and colonial morphologies. |
|
Although a Gram-positive rod, Erysipelothrix rhusiopathiae may stain Gram-variably. Microscopic examination of a colony of E. rhusiopathiae reveals short, slender, slightly curved bacilli, sometimes forming long filaments. A mixture of both rough and smooth colonies may found on culture, with colonies small, circular, and transparent; they may be alpha-hemolytic after prolonged incubation (>48 hr). Growth may be enhanced under microaerobic conditions. E. rhusiopathiae is catalase-negative and also (slowly) produces H2S on triple sugar iron (TSI) agar, which is helpful in differentiating it from other Gram-positive bacilli. If what appears to be Lactobacillus is present in a high quality specimen (e.g., blood culture) or in a cutaneous or subcutaneous wound, then a TSI slant should be innoculated with the organism to exclude E. rhusiopathiae. Although its cells fail to palisade, this organism might conceivably be confused with a coryneform, but its lack of catalase activity would be an unexpected finding. |


