|

Unstretched rubber

Stretched rubber
|
One
of the aspects of balloons that make them so beautiful to look at is their
shine. A properly inflated balloon, no matter the brand or color, will
always have a glistening shine to it. Furthermore, the tighter a balloon
is inflated, the shinier it becomes.
The
reason a material shines is that it reflects light coherently, that is,
two parallel light rays remain parallel after being reflected. Mirrors
reflect light in this way, as does glass, and any smooth metal. All these
materials have a surface that is uniform and smooth, allowing reflected
light to remain parallel. Rough surfaces scatter light in many different
directions, making them poor reflecting material.
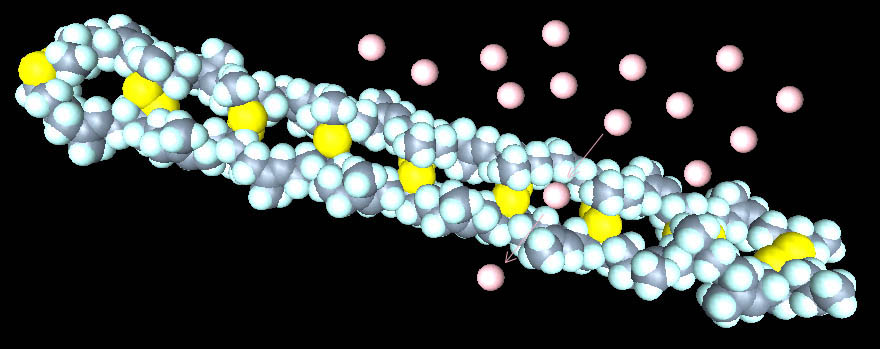
Rubber
that is not stretched, such as that of an uninflated balloon, is highly
coiled and presents an uneven, rough surface for light that scatters
incoming light rays and makes the rubber appear soft (above left-- click
for a better view).
Stretched
rubber, such as an inflated balloon, elongates the rubber molecule and
making it straighter. This straighter, smoother surface reflects light
very well. In particular, the tighter the balloon rubber is stretched, the
straighter the rubber molecules and the better the reflection of incoming
light, making overinflated balloons much shinier than underinflated ones.
However,
keep an inflated balloon around long enough and it will lose its shine--
partially because it shrinks (see above), but mostly due to the oxidation
of the rubber by molecular oxygen in the air (see Oxidation).
This puts a layer of oxidized rubber on the outside of the balloon, and
since oxidized rubber is chalky and molecularly rough, the light can no
longer be reflected as coherently as in a new balloon. |