

|
The cumulative research directed towards understanding the molecular biology underlying all aspects of disease & development has yielded a wealth of information. As gene products & their interactions with the cellular environment have been characterised, so the possibility of treating disease by using DNA as a drug has arisen. All proteins are coded for by DNA, & diseases ultimately result from the expression of one or more abberent proteins, e.g. an oncogene or pathogen protein, or the lack of a functional form. In theory therefore, all diseases could be treated by expression of the appropriate protein in the effected cells. Conceptually the most simple disease to treat would be a monogenic recessively inherited disease, such as haemophilia , whereby the functional form of the gene would be added to the cell restoring it to a normal phenotype. However, research is underway to treat monogenic dominantly inherited diseases such as hypercholesteroleamia , & acquired genetic diseases such as cancers. Regulation of cellular proliferation e.g. to prevent atherosclerosis following angioplasty , or to promote of cellular repair following trauma to the CNS & protection from infectious disease are also currently being investigated. Gene therapy potentially represents one of the most important developments to occur in medicine, but before this can be realised certain technical problems common to all methods of gene delivery must be overcome. In order to modify a specific cell type or tissue, the therapeutic gene must be efficiently delivered to the cell, in such a way that the gene can be expressed at the appropriate level & for a sufficient duration. Two broad approaches have been used to deliver DNA to cells, namely viral vectors & non-viral vectors, which have different advantages as regards efficiency, ease of production & safety. This paper will review these methods & then discuss the genetic strategies used to achieve prolonged tissue specific expression of the therapeutic gene.
PHARMACOLOGICAL CONTEXT OF GENE THERAPY
DRUG THERAPYAlmost all drugs act by altering the phenotype of the target cell, and may therefore be called phenotypic drugs. Among currently marketed drugs, the only exceptions to this rule are certain antineoplastic agents and anthelminthics. When pharmacological agents alter the genetic make-up of the cell, they may be called genotypic drugs. Almost all gene therapy falls into the category of genotypic pharmacology.
PHARMACEUTICAL DEVELOPMENTTraditional drug development has depended on the production of phenotypic agents. The success of the pharmaceutical industry in the past 50 years has depended on innovation in the discovery of new mechanisms of selective effect on phenotype. The time-honored formula has been:
DOWNTRENDS IN TRADITIONAL DRUG DISCOVERYThere has been a slowing of phenotypic drug discovery in the pharmaceutical industry in the past decade. This coincided with, and drove, recognition of the potential of genotypic agents to achieve greater specificity, potency, and versatility than was possible with previous modes of therapy. However, the importance of moving from exclusive reliance on phenotypic agents was not immediately apparent to all pharmaceutical firms.The largest and historically most successful ones were, in general, the slowest to react.
BIOTECHNOLOGY FIRMSThe gap created by this discontinuity encouraged the development of start-up biotechnology firms, some little more than a lab, a scientist, and a venture capitalist. Most biotechnology firms found they were good at innovation, but not very good at developing and marketing a lead compound. Many went bankrupt, but others developed lucrative relationships with traditional pharmaceutical companies, which possessed the development and marketing capabilities that the start-up companies lacked. The period 1994-1996 has been a time of major realignment associated with absorption of biotechnology firms by, or development of joint ventures with, traditional pharmaceutical firms. Thus gene therapy, pioneered in academic medical centers and the National Institutes of Health, followed its enthusiasts first into start-up biotechnology firms, and ultimately into the mainstream of the traditional drug industry.
BASIC REQUIREMENTS FOR GENE THERAPYPOTENTIAL OF GENE THERAPYGene therapy offers a new treatment paradigm for curing human disease. Rather than altering the disease phenotype by using agents which interact with gene products, or are themselves gene products, gene therapy can theoretically modify specific genes resulting in disease cure following a single administration. Initially gene therapy was envisioned for the treatment of genetic disorders, but is currently being studied in a wide range of diseases, including cancer, peripheral vascular disease, arthritis, neurodegenerative disorders and other acquired diseases.
GENE IDENTIFICATION & CLONING.Even though the range of gene therapy strategies is quite diverse, certain key elements are required for a successful gene therapy strategy. The most elementary of these is that the relevant gene must be identified and cloned. Upon completion of the Human Genome Project, gene availability will be unlimited, but until then the starting point for any gene therapy strategy remains gene identification and cloning for relevant genes related to the disease.
GENE TRANSFER & EXPRESSION.Once the gene has been identified and cloned, the next consideration must be expression. Questions pertaining to the efficiency of gene transfer and gene expression remain at the forefront of gene therapy research. Currently much debate in the field of gene therapy revolves around the transfer of desired genes to appropriate cells, and then obtaining sufficient levels of expression for disease treatment. Hopefully, future research on gene transfer and tissue-specific gene expression will resolve these issues in the majority of gene therapy protocols. Other important considerations for a gene therapy strategy include: a sufficient understanding of the pathogenesis of the targeted disorder, potential side effects of the gene therapy treatment, and understanding of the target cells to receive the gene therapy.
TERMINOLOGYLike most fields, gene therapy has unique terminology. The list provided below will clarify the meaning of some of the most common terms.

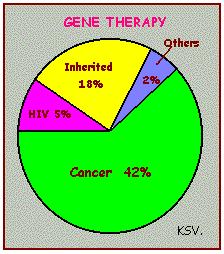
CURRENT STATUS OF GENE THERAPYAPPROVED PROTOCOLS Currently there are at least 150 clinical gene therapy protocols worldwide. Since the approval process for these protocols is not as public outside the U.S., it is difficult to obtain an exact number of worldwide protocols. Of the publicized protocols, 125 are approved in the United States, 48 in Europe, and at least 1 each in China and Japan. As of 31 December 1995, 1024 patients had been treated in either a gene transfer or gene therapy protocol. Much controversy exists regarding how many of these have benefitted from their gene therapy, and no one has yet been cured. PROTOCOL DIVERSITYOf the 125 RAC approved protocols, 25 are marker protocols and 100 are therapy protocols. Marker protocols can be distinguished from therapy protocols in that a marker protocol transfers a gene into cells for the sake of identifying those cells. Therapy protocols, on the other hand, involve the transfer of genes to cells, with the goal that expression of the transferred gene will ameliorate disease. The majority of the therapy protocols focus on treating acquired diseases such as cancer or HIV. Inherited disorders are the focus of 22 gene therapy protocols which are aimed at treating 9 different genetic diseases. Three other gene therapy protocols round out the list of 125. These are aimed at treating peripheral vascular disease, rheumatoid arthritis, and arterial restenosis.
 CANCER PROTOCOL STRATEGIES Cancer gene therapy protocols employ a wide variety of strategies and can be grouped as follows: in vitro insertion of a cytokine gene into tumor cells; in situ injection of an HLA gene; in situ insertion of a suicide gene into tumor cells; use of tumor suppressor genes or anti-on cogenes; and use of the multidrug resistant gene. GENE THERAPY VECTORSThe majority of these 125 RAC approved protocols employ retroviral vectors (63%) to deliver the selected gene to the target cells. Other widely used vectors include adenoviral vectors (16%), liposomes (13%) and adenoassociated vectors (2%). The remaining 6% employ a variety of vector systems, the majority of which include injection of naked plasma DNA. Among human gene therapy protocols at Vanderbilt, the oncology protocols (Jeff Holt and collaborators) employ retroviral vectors and the pulmonary protocols (Ken Brigham and collaborators) employ a liposome strategy.
Public controversy in the field of human gene therapy is driven by several factors. The enormous potential of gene therapy is easily understood by ordinary citizens as well as scientists, but the former may not appreciate all the pitfalls and uncertainly that lies in the immediate future. The financial interests of biotechnology firms and, some have asserted, the career interests of some gene therapists have encouraged extravagant, or at least overly optimistic, public statements about contemporary gene therapy. In spite of the proliferation of protocols, the actual number of patients treated remains small, and only one genuinely controlled study of human gene therapy has been published as of this date.
VIRUSES AS VECTORSViruses are obligate intra-cellular parasites, designed through the course of evolution to infect cells, often with great specificity to a particular cell type. They tend to be very efficient at transfecting their own DNA into the host cell, which is expressed to produced new viral particles. By replacing genes that are needed for the replication phase of their life cycle (the non-essential genes) with foreign genes of interest, the recombinant viral vectors can transduce the cell type it would normally infect. To produce such recombinant viral vectors the non-essential genes are provided in trans, either integrated into the genome of the packaging cell line or on a plasmid. As viruses have evolved as parasites, they all elicit a host immune system response to some extent. Though a number of viruses have been developed, interest has centred on four types; retroviruses (including lentiviruses), adenoviruses, adeno-associated viruses & herpes simplex virus type 1.
|

 |