The plasma membrane (PM) encloses the cell, controls the passage of materials into and out of the cell and contains proteins essential to cell adhesion, recognition and communication.
Other membranes enclose cellular organelles with specific functions (endoplasmic reticulum, golgi, mitochondria, nucleus, etc.).
Permit cellular and sub-cellular compartmentation and specialisation of function.
May maintain and use ion and other gradients (e.g. for action potentials - excitable cells, ATP synthesis - Mitochondria).
Carry enzymes/proteins vital to the life of the cell (ribosomes, Na+/K+ ATPase, cytochromes, transport proteins,, hormone receptors, etc.).
Biological membranes consist of water (~20% by wight), lipid, protein and carbohydrate. The relative proportions of lipid and protein can vary greatly: myelin is 80% lipid, whereas the inner mitochondrial membrane is 25% lipid.
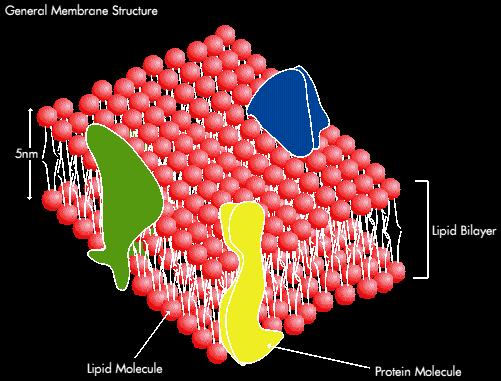
The basic structure of biological membranes consists of a lipid bilayer containing a variety of embedded or attached membrane proteins:

 Lipids constitutes around 50% of the mass os most animal cells; there are around 5 million lipid molecules in one square micron of membrane (or around 109 molecules per cell.). The Major lipid components are phospholipids, cholesterol and glycolipids. In membranes the phospholipid are often unsaturated.
Lipids constitutes around 50% of the mass os most animal cells; there are around 5 million lipid molecules in one square micron of membrane (or around 109 molecules per cell.). The Major lipid components are phospholipids, cholesterol and glycolipids. In membranes the phospholipid are often unsaturated.
Cholesterol can be equimolar with phospholipids and is an important fluidity regulator (discussed later). It consists of a rigid platelike ring portion and a more flexible hydrocarbon tale which penetrates the bilayer to about the level of a 14C fatty acid. Plant membranes contain other sterols in place of cholesterol.
Which have covalently attached carbohydrate moieties and are concentrated in the outer layer of the PM (eg. the sialic acid containing gangliosides of nerve cells).
In animal cells, glycolipids are produced from ceramide, a precursor of sphingomyelin.
They are thought to have roles in cell adhesion and recognition (cholera toxin binds to GM1, regulation of electrical conductance, myelin as well as a protective function (against enzymes, low pH, etc.).
Although the bilayer structure of membranes is determined by its lipid components, all membranes also contain protein (up to 75% by mass in some complex enery transducing membranes - e.g. mitochondria).
Many membrane proteins have covalently attached oligosaccharide chains. On the cell surface this forms a carbohydrate cell coat or "glycocalyx".
These are amphipathic, having hydrophobic regions which span the lipid bilayer, usually (but not always) as an alpha-helix. Hydrophilic regions extend on either side of the membrane and may connect a number of mebrane spanning domains. Membrane spanning regions in an AA sequence may be identified by hydropathy plots.

In the above hydropathy plot the membrane is crossed once by the protein, in the below one the membrane is crossed 7 times.

Some transmembrane proteins may cross the membrane as a closed ß-sheet structure (ß-barrel). Examples are bacterial porins which allow entry of molecules less than around 600M.W.
Transmembrane proteins may be visualised by freeze-fracture electron microscope, where the surfaces of a membrane are coated with Pt.
These may be linked to a fatty acid or other lipid (prenyl) group which forms part of the lipid bilayer; alternatively they may be linked to an oligosacharide, covalently linked to a phosphatidiylinositol, forming a glycosylphosphatidylinositol (GPI) anchor.
Some proteins are attached to the membrane by non-covalent interactions with other membrane proteins. These may be extracted by relitavely gentle techniques (eg salt washing, pH)
Many of the properties of membranes depend upon the property of the proteins and lipids within it to diffuse freely within the lipid bilayer. This property is called membrane fluidity. Fluidity may be regarded as a measure of the viscosity of the membrane.
Phospholipid mobility within the membrane may be intra- or inter molecular. Intramolecular movement may be:
Phospholipid molecules do not readily "flip-flop" from one side of the bilayer to the pother (less than once a month per molecule!). In the ER enzymes called "phospholipid translocators" catalyse flip-flop of newly synthesised molecules.
Intermolecular motion is lateral diffusion of the whole phospholipid molecule. Lipid molecules exchange places with their neighbours ~107 sec-1 (=diffusion coefficent of ~10-8 cm2 sec-1, a lipid molecule can diffuse the length of a 2µ cell in 1 second!).

Phospholipid bilayers can exist in a rigid gell or liquid christalline state depending on the temperature. The "Phase Transition" between these two states can be detected by Differential Scanning Calorimetry, which measures the relationship between rise in temperature and heat uptake, an dthus detects the latent heat associated with phase transition.

In the gel state, acyl chains are fully extended and tilted to the plane of the bilayer. At pre-transition the bilayer is disordered by periodic ripple. Above the transition temperature, acyl chains beocmoe distorted so that instead of an all "trans" arrangement around each C-C bond, "gauche" isomers appear. This change can be detected by Raman spectroscopy which detects the stretching vibration around C-C bonds.

This is largely as a result of their cholesterol content, which can be up to 50% on a molar basis. The rigid, planar steroid rings interact with and imobilise those regions of the fatty acid chains that are closest to the surface of the membrane. This contributes towards making the lipid bilayer less fluid and reduces it's permeability to small molecules. However, in addition to reducing fluidity, it also inhibits the packing of the fatty acid tails and therefore inhibits possible phase transitions - this is infact what leads to the "gel" state.
Membrane proteins are also able to move within the bilayer
Membrane proteins undergo rotational diffusion (about the protein axis, perpendicular to the bilayer), and lateral diffusion(within the membrane). Like membrane proteins they do not "flip-flop" across the bilayer.
This lateral diffusion is of great functional importance, e.g.collision coupling model of signal transduction across the PM and Patching/Capping of membrane proteins which bind multivalent ligands such as antibodies.
eg. A fluorescent monovalent antibody to a specific protein:

Measurements like this indicate that membrane proteins have lateral diffusion rates of approximately 1 to 10% of that of lipids
Some Proteins are Confined to Specific Domains
For example, in epithelial cells, certain transport proteins and PM enzymes are confined to the apical surface by "tight junctions", which are areas of the outer PM leaflet of adjacent cells tightly sealed together, probably via specific junctional proteins.
Tight junctions also restrict the diffusion of lipids in the outer leaflet of the bilayer to particular membrane domains and therefore contribute to the maintenance of membrane asymmetry.
Cells also restrict the movement of membrane proteins by attaching them to macromolecular assemblies either outside or inside the cell, for example "band3", a transmembrane protein responsible for bicarbonate transport in red blood cells is attached via a protein called ankyrin to the cytoskeletal network responsible for maintaining cell shape. Cells can also restrict movement by assembling the proteins into large aggregates within the membrane which form two dimensional crystals incapable of rappid diffusion. Eg. Bacteriorhodopsin in Halobacterium.
As a result od these retrictions on the mobility of some membrane proteins, diffusion coefficints measured for a range of membrane proteins vary considerably.

The lipid bilayer itself is asymmetric with respect to both lipids and proteins
| Sealed Right-Side-Out Ghost | leaky Ghost | Intact Cell | Leaky Inside-Out Ghost | Sealed Inside-Out Ghost | |
| Transmembrane | Yes | Yes | Yes | Yes | Yes |
| Extracytoplasmic | Yes | Yes | Yes | Yes | No |
| Cytoplasmic | No | Yes | No | Yes | Yes |
| Intramembrane | No | No | No | No | No |
The asymetric orientation of transmembrane proteins reflects their physiological rolse and originates in their co-transitional insertion into the membranes, determined by "signal sequences".
Enzymes in the ER can move proteins across the bilayer
PC and SPM are in the outer leaflet;
PE, PS and PI predominate in the inner leaflet;
Cholesterol is also distributed preferentially to the outer leaflet.
PL's are also distributed asymmetrically in the intracellular membranes.
eg. The concentration of PS in the inner leaflet of the PM leads to significant cgarge difference between the two urfaces.
Phosphatidylinositol 4,5-bisphosphate is found on the inner surface of the PM where it is cleaved by a receptor-activated enzyme to yeild two intracellular messenger molecules involved in signal transduction.
PS in the inner leaflet binds protein kinase C during signal trasduction.
Individual cells are organised into tissues where they make contact with each other and with the extracellular matrix (a range of secreted macromolecules). Cells have particular structures (cell junctions which maintain contact both with the extracellular matrix and with adjacent cells. The relative importance of these two types of interaction depends on the tissue. In connective tissues, with relatively few cells, cell matrix interactions perdominate. In epithelial cells, which form a barrier lining all body cavities and surfaces, cell-cell contacts are crucial and those with theextracellular matrix (the basal lamina) less important.
Cell junctions may be classified according to their cellular roles
Tight junctions effectively form a permeability barrier across epithelial barrier across epithelial cell surfaces. This has two vital roles:
In the gut epithelium for example glucose is taken up ftom the gut lumen by Na+ symport at the apical suirface and leaves the vell by facilitated diffusion via specific glucose carriers n the basolateral membrane. Tight junctions prevent these membrane proteins moving to the wrong side of the cell and also prevent glucose seeping back between the cells.
Although all tight junctions are impermeable to macro-molecules, their permiability to small molecules varies from cell to cell: eg. The gut epithelium is 103 fold more leaky to small ions that is that of the urinary bladder.
Epitherlial cells can regulate the permeability of their tight junctions tosome extent: Eg. amino acids and monosacharides can pass directly from the lumen of the gut to the blood when the concentration gradient is sufficient (paracellular transport).
Structure of Tight Junctions
Current views of tight junctional structure are based on electron microscopy. Continuous strands of transmembrane junctional proteins in adjacent ceslls are thought to contact and make a seal across the intracellular space.
Thus group of junctions conect the cytoskeleton to the exxtracellular matrix of neighboring cells. They maintain the structural integrity of groups of cells and issues and are especially abundant in tissues which are subject to mechanical stress (for example the heart muscle and skin).
All anchoring junctions share common structural characteristsc. Extracellular Attachment Proteins form a plaque on the inner surface of the PM and connect to eiter actin filaments or intermediate filaments. Transmembrane linker proteins bing intracellular attachment proteins on the cytoplasmic side of the plasma membrane and interact with either extracellular matrix proteins or transmembrane linker proteins from other cells on the extracytoplasmic surface.

In non epithelial cells, these connect cytoplasmic actin filaments to adjacent cells. In epithelial sheets, they form a continuous adhesion belt on the basolateral side of the tight junctions.
Within each epithelial cell, bundles of actin filaments run parallel to the junctional PM. These are attached by intracellular attachment proteins like catenin and vinculin to Ca2+ dependent transmembrane linker proteins called adherins. The extracellular domains of cadherins on adjacent cells bind each other to form the junction. Hence the actin bundles of epithelial cells are linked inot a transcellular network or "adhesion belt". The contraction of this network is vital to morphogenisis (eg. in the formation of epithelial tubes like the neural tube).
Cell-matrix adherens junctions are points of contact (adhesion plaques) between cells and the extracellular matrix. The transmembrane linker proteins involved in these junctions are membranes of the integrin family of cell surface matrix receptors. A variety of attachment proeins ink the extracellular domains of the integron receptors to the actin filaments.
Desmosomes are intercellular junctions which join cells together, thus confering resistance to shearing forces and tensile strength. Desmosomes consist of a cytoplasmic plaque of intracellular attachment proteins which link th eintermediate filaments of adjacent cells to transmembrane linker proteins of the cadherin family of Ca2+ adhesion proteins.
Pemphigus is a serious skin disease in which auto-antibodies against desmosome cadherins of keratinocytes causes skin blistering and loss of flud through the skin.
Hemidesmosomes are similar in structure to desmosomes but they connect the basal membrane of epithelial cells to the basal lamina at the interface between the epithelium and the connective tissue. The transmembrane linker proteins are integrins (as in adhesion plaques). Thus integrins are always the trasmembrane linker proteins which bind the extracellular matrix whilst cadherins mediate cell-cell adhesion. All these junctions couple to cytosskeletal elements (either actin or intermediate filaments) and all are dependent on extracellular divalent cations.
Gap Junctions
On EM these appear as regions of cell contact where neigbouring plasma membranes (PMs) are separated by 2-4 nm. Gap junctions are spanned by poreforming proteins which allow ions and small molecules (<1000 Da) to pass from cell to cell. Hence the cytoplasm of adjacent cells is metabolically and electrically coupled. This is of conciderable significane in cell cell communication, both in electrically excitable tissues and other cells.
Gap junctions are composed of six identical transmembrane protein subunits called connexins which together form a connexon. Each connexin has 4 membrane spanning alpha helicies, one of which lines the central pore. Antibodies to connexins block gap junction permeability and the mRNA encoding the protein induces gap junctions in cells which do not normally posses them. The permeability of gap junctions is diminished by low pH or increased [Ca2+].

This permeability change is thought to e mediated by a small rotation of each connexin and is vital in isolating damaged cells from thir neighbors. There are multiple connexins in animal cells, thus explaining the variable permeability of gap junctions in different cells.
Gap Junctions
these are a specialised form of communicating junction which occur between nerve cells and other nerve cells or muscle cells. The pre-synaptic membrane of the proximal nerve terminal is spearated form the post synaptic membrane of the communicating nerve of muscle cellby a ynaptic cleft of ~50nm.

An action potential in the pre-synaptic nerve depolarises the pre-synaptic membrane, opening volyage-gated Ca2+ channels. The rise in intracellular [Ca2+] causes synaptic vesicles to fuse with the pre-synaptic membrane and release neurotransmitter (eg. acetylcholine) into the synaptic cleft. The neurotransmitter diffuses across the synaptic cleft. In the case of acetylcholine, it binds to specific receptors in the post synaptic membrane which mediate an influx of Na+ causing local depolarsiation and initiating another action potential. If the synapse terminates on a muscle cell, the depolarisation associated with the action potential opens voltage sensative Ca2+ channels and causes Ca2+ release from the sarcoplasmic reticulum. It is this increase in [Ca2+] which causes the myofibrils to contract.
Plant cells are surrounded by rigid cell walls made of extracellular matrix rich in cellulpse. These effectively anchor adjacent cells together, but plant cells still have communicating junctions called plasmodesmata. These connect the cytoplasm of adjacent cells through the cell wall, thereby spanning a gap of about 100nm (cf. 2.4nm for gap junctions).
Plasmodesmata are cytoplasmic channels of 20 - 40 nm diameter containing a desmotubule which connects the smoothe ER of the adjoining cells. Like gap junctions they allow the passage of molecules of M.W. <800Da and some plant viruses also pass through them. Their permeability can be regulated. However, little is known of the proteins or mechanisms involved.
Eukaryotic cells take up a variety of substances from their environment. The process, which is known as endocytosis, delivers material to specialised compartments within the cell caled endosomes and lysosomes. It may serve several purposes:
Endoxytosed molecules are sorted during the process so that they can be sent to different compartments of the cell.
We generally distinguish true endocytosis, which can be either fluid phase (pinocytosis) or receptor-mediated from phagocytosis.
| Phagocytosis | Endocytosis | |
| Stimulated | Constitutive | |
| Particle Size | >250nm | <150nm |
| Cells | Certain | All |
| Internalisation | Preceded by Binding | With or Without Binding |
| Actin Mediated? | Yes | No |
| Delivered to Lysosomes? | Always | Not Always |
Endocytosis
May be receptor mediated of fluid phase (pinocytosis), both are initated in specialised regions of the plasma membrane called clathrin coated pits.
Pinocytosis
All eukaryotic cells constantly ingest small vesicles of plasma membrane containing extracellular fluid (endocytotic vesicles). After processing the dissolved contents, the mebrane returns to the PM as part of an endocytotic/exocytotic pathway. Macrophages can ingest 25% of their volume by pinocytosis every hour, recycling their entire PM twice in the process!
Clathrin, which is essential to all forms of true nedoytosis, consists of 3 heavy chains and 3 light chains, forming a tree legged trimer called a triskelion. In the presence of Mg2+ and a pH of <7, these trimers can be shown to form a closed polyhedron. It is this tendancy to polymerise on the cytosolic side of the pM which causes coated pits to pinch off inside the cell and form clatherin-coated vesicles (Ø ~ 80nm).
The clathrin shell of coated vesicles is removed by a "heat shock protein" which is an ATP driven uncoating enzyme. It costs 3 ATP's to remove each tri-skeleion, illustrating the thermodynamic stability of the clathrin coat. The coated vesicles then fuse with the early endosomes (Ø ~ 200 - 600nm) and the clathrin subunits re-associate with the PM. It takes ~20 seconds for a coated pit at the plasma membrane to seal off and travel to the endosomal compartment. In cultured fibrobalsts, 2500 coated vesivles leave the PM per minute.
 "Early" and "Late" Endosomes
"Early" and "Late" Endosomes
The endosomal compartment is a complex arrangement of heterogenous tubes and vesicles extending from the PM to near the nucleus. Early endosomes, which accumulate extracellular markers in approximately one minute, are distinguished from late endosomes, which are labelled after five to fifteen minutes.
Two theories exist to explain the passage of endocytosed material between the endosomal compartments. Early endosomes may merely migrate towards the nucleus to become late endosomes which fuse woth hydrolase-containing vesicles from the trans Golgi to become lysosomes. Alternatively, transport from early to late endosomes may be mediated by endosomal carrier vesicles which migrate along microtubules. In either case,a separate vesicular system can exchange material between late endosomes and the trans Golgi.
Receptor Mediated Endocytosis
Specific macro-molecules can also be endocytosed via clathrin coated pits. Such macromolecules bind to complementary transmembrane recetor proteins. receptor mediate endocytosis performs a number of viatal physiological functions:
Membrane recpetors mediating receptor-mediated endocytosis are usually glycoproteins with a large, glycosylated extracellular domain, 1 or 2 transmembrane helices and a small cytosolic region.
Receptors for some ligands (eg. low density lipoprotein, transferin and insulin) accumulate in coated pits whether or not they are occupied, Oters (eg. EGF receptor) only cluster in the ligated state.
Clathrin coated vesicles rich in a prticular receptro are formed via a second major coat protein called adaptin
Adaptins recognise the cytosolic domains of various recoeptor proteins and serve as linker molecules between these receptors and the clathrin coat, thus capturing specific cargo molecules within the coated vesicle. This mechanism allows the concentration of particular ligands in coated vesicles by many orders of magnitude (cf. the concentration of their receptors in the PM) and allows them to be targeted within the cell.
Coated pits appear to be able to act as molecular filters and exclude certain membrane proteins
Cells that require cholesterol up-regulate LDL receptors in their PM where they migrate and associate with clathrin coated pits. The tendancy of clathrin to form closed polyhedral lattices ensures that the area of the PM rich in LDL receptors (which will carry bound LDL) is internalised in a coated cesicle. Having shed the clatrin coat, these vesicles fuse with early endosomes near the PM. Here, LDL dissociates from it's receptor in the acidic environment (pH 6, due to ATP driven H+ pump).
The empty LDL receptros are returned to the PM via transport vesicles that bud off from the tubular region of the early endosomes. The time taken for an LDL receptor to complete this cycle and return to a new coated pit is about 10mins.
LDL moves via late endosomes, where he cholesterol esters become mixed with newly-synthesised acid from the <>transgolgi, to the lysosomal compartment and are hydrolysed to free cholestero,, which is then available for membrane syntheisis.
When too much free cholesterol accumulates in a cell, it shuts down both it's own cholesterol synthesis and the synthesis of LDL receptors.
Defects in receptor mediated endocytosis (usually caused by some defect in the LDL receptor) occur inabout 1 in 500 individuals. Souch defectsl lead to hypercholesterolaemia which causes atherosclerotic plaques to dorm in the arteries and provoke premature heart attackes.
There are at least four classes of familial hypercholesterolaemia:
This latter class may be caused by a mutant LDL receptor which has amino acid (AA) substitutions in the ~50AA's which comprise the cytoplasmic tail of this single pass transmembrane glycoprotein. Such mutant receptors cannot bind to the adaptins which facilitate the formation of the clathrin coat.
Transferin transports iron into cells. The iron-free protein is called apotransferrin and binds two Fe3+ per 77kd protein. Transferrin, but not apotransferrin, binds to a dimeric receptro in coated pits and is internalised to early endosomes where the low pH reduces the affinity of transferrin for Fe3+ by 106, causing it to dissociate. Apotransferrin remains bound to the receptor at this low pH and is returned to the pM, where the sudden shift in pH (~7.4) causes it to be released into the extracellular medium to repeat the cycle. This whole cycle takes about 16 minutes and a liver cell can take up 20,000 Fe3+/hour in this way.
in the immune system, helper T cells are activated by specialist antigen presenting cells (eg. interdigitating dendric cells, B cells and macrophages). These cells ingest invading organisms or their products by receptor mediated endocytosis and partially degrade them in the acidic environment of the late endosome. This compartment also recies classII majot histocompatibility complex (MHC) proteins from the Golgi which, at thius stage, have their binding sites, masked by an "invariant chain". This invatiant chain is degraded in the late endosomes, allowing the mHC protiens to bind the processes antigen. The antigen class II MHC complex is delivered to the plasma membrane by transport vesicles, where it is recognised by helper T cells.
The early endosomal compartment performs the main sorting fnction for endocyosed proteins (cd. trans Golgi in exocytosis). All endocytosed ligands that dissocitae from their receptors in early endosomes are destined for destruction in the lysosomes; howeverm receptors and ligands that remain receptro-bound , have a variety of potential fates:
This allows them to recylce endocytosed recpetors to their original PM domains unless they contain transcytotic signals (eg. maternal IgG/IgA). Molecules that are not retrieved frin the early endosomes by one of these routes pass to a common late endosomal compartment & thence to lysosomes.
Influenza and yellow fever viruses enter this way, but the life cycle o the Semliki Forest Virus (SFV) is best understood:
Diptheria toxin is a bacterial toxin which is internalied by receptor-mediated endocytosis after binding to a receptor on the cell surface whch is a grwoth factor pre-cursor. Once inside the endosome, the toxin is cleaved into a 21kd ‘A’ fragment and a 40kd ‘B’ fragment. A membrane insertion damin on the B fragment is activated by the acidic environement and this fascilitaies the passage of the A fragment from the lysosome to the cytosol.
The A fragment is an enzyme which catalyses the transfer of ADP-Ribose from NAD+ to the elongation factor EF2, leading to the complete block of protein syntheis and cell death.
A Zip file of some OHP's (produced by and © me) in Draw format is available here:Dipy.zip. This is only suitable for Acorn machines, and will be absolutely useless to PC users, including those with Xara as it's all text and sprites!
