

Biological Membranes are organised sheet like assemblies consisting mainly of lipids and proteins. They are highly selective permeability barriers - but not impervious - they have specific channels and pumps which regulate the molecular and ionic composition of the intracelluar medium
Eukaryotic Cells also contain internal membranes which delinate the boundaries of organelles such as mitochondria, chloroplasts, and lysosomes. Functional specialisation during evolutions has been linked to the formation of such compartments. Membranes also control the flow of information between cells and their environment by having more specific receptors for external stimuli; some membranes also generate signals which can be either chemical or electrical - as in the transmission of nerve impulses.....Membranes are complex structures!
Most of the membranes' phospholipid and glycolipid molecules are arranged in a bilayer which has a dual role (it is a solvent for integral membrane proteins and it also acts as a permeability barrier). But a small proportion of membrane lipids interact specifically with particular membrane proteins which is a function which may be essential for their function.
Membrane proteins are free to diffuse laterally within the lipid matrix unless they are restricted by specific interactions however they are not free to rotate from one side of the membrane to the other.


Integral membrane proteins interact extensively with the hydrocarbon region of the bilayer - nearly all of them traverse the lipid bilayer; Peripheral membrane proteins bind to the surface of integral membrane proteins.
The three types of protein synthesised are:
If free ribosomes from the cytosol are isolated and then added to rough endoplasmic reticulum which has been striped of its' ribosomes - the reconstituted system actively synthesises proteins when presented with mRNA and soluble factors.
The same effect was true for ribosomes isolated from rough endoplasmic reticulum which were fully active in synthesising proteins normally released into the cytosol.
There are no structural differences between free ribosomes and those from rough endoplasmic reticulum (E.R.). I.e. Membrane bound and Free Ribosomes are intrinsically identical, whether a particular ribosome is free or attached to rough E.R. depends only on the kind of protein it is making.
Attachment of an actively synthesising ribosome to E.R. membrane is a key event in translocating a protein across the membrane.
The Signal Hypothesis was proposed in 1970 and stated that:
"The Signal for attachment was a sequence of amino acid residues near the amino terminus of the nascent polypeptide chain"
This hypothesis was soon supported by experimental evidence; it was shown that major secretory proteins (e.g. proteins of the pancreas) contain amino-terminal extensions of about 20 residues when synthesised in vitro by free ribosomes.
These signal sequences - absent from normally secreted protein - as they are cleaved by a signal peptidase on the lumenal side of the E.R. membrane.
Substitution of a charged residue for a non-polar one in this hydrophobic core destroys the directing activity of the signal sequence.
Not all secretory and plasma membrane proteins have an amino terminal signal sequence that is cleaved following translocation across the e.r. membrane - some have an internal signal sequence (e.g. ovalbumin).
By experimental manipulation is is possible to alter a cytosolic protein to be redirected to the E.R. - this is done by joining a signal sequence to it's amino terminal.
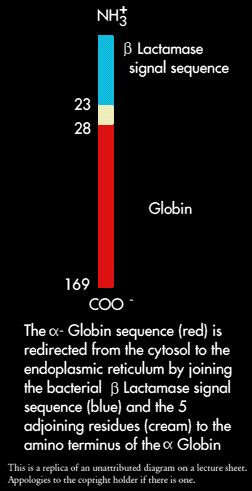
The E.coli enzyme ß0Lactamase, that hydrolyses penicillin is secreted into the periplasmic space between the outer and plasma membranes as it is synthesised. In the process a 23 residue signal sequence is cleaved from the polypeptide.
This sequence and 5 adjoining residues of ß-Lactamease were joined to the amino terminus of the alpha-chain of haemoglobin by forming a hybrid gene. Alpha-globin was used because it normally stays in the cytosol and is highly hydrophilic.
 The m-RNA of this chimeric protein was added to an in-vitro protein synthesising system that can translocate secretory proteins across the E.R. membrane. This system consisted of four major components:
The m-RNA of this chimeric protein was added to an in-vitro protein synthesising system that can translocate secretory proteins across the E.R. membrane. This system consisted of four major components:
Ribosomes synthesising alpha-globin with a signal sequence became bound to microsomes and the nascent polypeptide chain traversed the endoplasmic reticulum membrane. The signal sequence was cleaved and the alpha-globulin was sequestered inside the vesicle. In other words, addition of a signal sequence to alpha-globulin converted it from a cytosolic protein into a secretory protein.
The experiment also showed that bacterial and eucaryotic signal sequences are functionally similar.
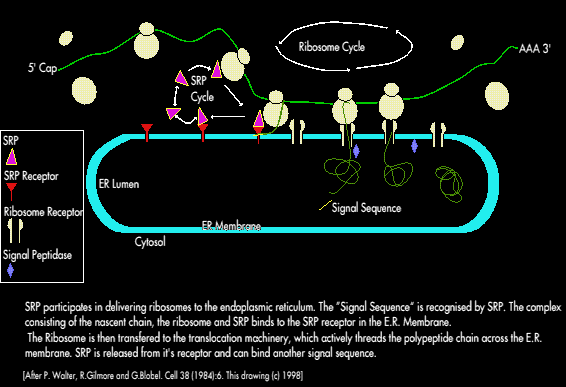
A Ribonucleic protein -called Signal Recognition Particle (SRP) couples protein synthesising machinery in the cytosol to protein translocating machinery in the E.R. membrane. SRP is a 325kD; 300 nucleotide RNA molecule of six different polypeptide chains.
SRP binds tightly to ribosomes containing a nascent chain with a signal sequence (but not to other ribosomes); this binding occurs soon after the emergence of the amino terminal signal sequence from the ribosome, elongation of the polypeptide is stopped or slowed while SRP binds. The SRP Ribosome complex binds to the E.R. membrane where SRP binds to the SRP receptor (called the docking protein).
A GTP driven process delivers ribosomes containing the nascent polypeptide chain to the translocation machinery. The concomitant release of SRP from ribosomes leads to the resumption of elongation i.e. SRP acts catalytically to deliver ribosomes with a signal sequence to the endoplasmic reticulum membrane.
SRP also prevents the premature elongation and folding of the nascent chain - which would interfere with translocation.
Many secretory and membrane proteins bear covalently attached carbohydrate moieties linked to either Asparagine side chains (by N-Glycosydic links) or Serine & Threonine side chains (by O-Glycosydic links). The N-linked oligosaccharides (though diverse) have a common pentasaccharide core of 3 mannose and 2 N-Acetylglucoseamine.
A larger common oligosaccharide block can be constructed on an activated carrier, which transfers it to a growing polypeptide on the lumenal side of the ER membrane. The carrier is Dolichol Phosphate - a long lipid molecule consisting of 20 isoprene (C5) units.
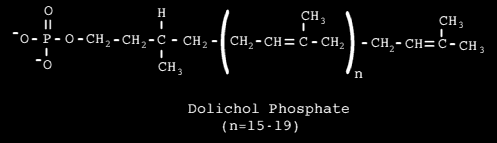
The terminal phosphoryl of GRP is the site of attachment of activated oligosaccharide. Proteins in th lumen of the ER and membrane are transported to the golgi complex (a stack of membranous sacks).
Transport of proteins between ER and Golgi, and golgi and subsequent destinations is mediated by small (50 - 100nm Ø) membrane bound compartments - called transport vesicles
The Golgi consists of 3 or 4 membranous sacs (cisternae) - the stack of cisternae is asymmetrical. The golgi is differentiated; there are three compartments:
Each compartment contains different enzymes and mediated distinctive functions. Different vesicles transfer proteins from one golgi compartment to another and then on to lysosomes, secretory granules or the plasma membrane.
Many cells have more than one secretory pathway:
Soluble proteins in the lumenb of the E.R. Emerge in constitutive secretory vesicles and are exported (unless special signals are present to export them to other locations). Similarly, integral membrane proteins, initally in the endoplasmic reticulum are exported to the plasma membrane unless they carry instructions to the contrary
Membrane lipids can be shown to be asymmetrically distributed by the use of phospoholipases which cannot pass through membranes, and therefore only phospoholipids on the external surfaces of intact cells are hydrolysed. The distribution appears to arise from membrane orientations of enzymes that synthesise phospholipdis and the tendency of ATP dependent phospholipid translocases to generate asymmetric phospholipid distributions.
Membrane Proteins are also asymmetrically distributed; this is shown by surface labelling (which uses agents that react with proteins but cannot cros the membrane). An example of this is the binding of an antibody elicited against it by the membrane of a cell, this could only bind to the inside of a membrane if the cell membrane were damaged.
Another method is to use membrane impermeable protein specific reagents that are labelled with either fluorescent or radioactive markers.
Using these techniques it has been seen that some integral proteins are exposed to a specific surface of a membrane or in the case of transmembrane proteins orientated in only one direction.
The asymmetrical orientation of integral membrane proteins is maintained by the infinitesimal "Flip-Flop" rates (which are slower even than that of lipids!).
The Cytosolic face of a transport vesicle corresponds to the cytosolic face of the donor compartment.
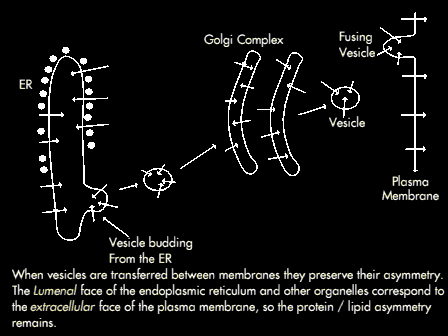
The topology of fusion mirrors that of budding following fusion, the cytosolic face of the transport vesicle becomes continuous with the cytosolic face of the target compartment. The consequence of this is that the lumenal side of transport vesicles and golgi correspond to the lumenal side of the ER membrane.
However during fusion of vesicles with the plasma membrane the lumenal surface becomes part of the extracellular surface of the plasma membrane (therefore the lumenal side of the ER and other organelle membranes corresponds to the externalface face of the plasma membrane. For this reason, the CHO groups of glycoproteins in the plasma membrane are always on the extracellular surface; in addition the lumenal contents of intracellular compartments are not released into the cytosol during budding and fusion.
Once a protein enters the endoplasmic reticulum it never returns to the cytosol.
Fusion following uncoating is blocked by N-Ethyl Malemide (NEM) which alkylates the -SH groups. This discovery lead to the isolation of NEM sensitive fusion factor - called NSF. ATP, fatty acyl CoA's and several other proteins are all required for fusion.
NSF is an ATPase; ATP binding is necessary for membrane attachment whereas, ATP Hydrolysis is required for release. It (NSF) also requires additional cytosolic proteins to bind to golgi membranes - these soluble NSF attachment proteins (or SNAPS) recognise a membrane receptor. Several SNAP receptors (SNARES) from the brain have been identified.
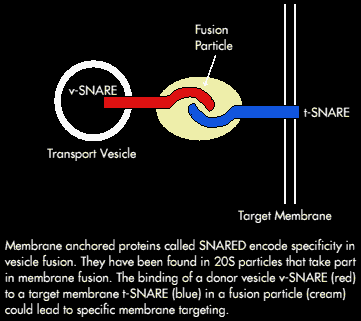 Syntaxin - a SNARE - is present in the presynaptic plasma membrane, adjacent to synaptic vesicles that are ready to fuse. The trigger for fusion is a rise in cytosolic Ca2+ caused by depolarisation of the nerve terminal.
Syntaxin - a SNARE - is present in the presynaptic plasma membrane, adjacent to synaptic vesicles that are ready to fuse. The trigger for fusion is a rise in cytosolic Ca2+ caused by depolarisation of the nerve terminal.
A working hypothesis is that the vesicles find specific targets by pairing of complementary receptors (i.e. a v-SNARE [from a transport vesicle] and a t-SNARE [from the target compartment]).
