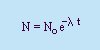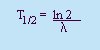Home Page of Peggy E. Schweiger
Nuclear Physics and Radioactivity
Structure and Properties of the Nucleus:
A nucleus can be considered to be made up of two types of particles--protons and neutrons.
- Proton
- The nucleus of the simplest atom, hydrogen. It has a positive charge of +q and a mass of mp = 1.67 x 10-27 kg.
- Neutron
- A particle found in the nucleus that is electrically neutral and that has a mass almost identical to the proton. It was discovered in 1932 by James Chadwick.
- Nucleons
- The term that refers to the two constituent particles of a nucleus.
- Atomic Number
- The number of protons in a nucleus (designated by the letter Z).
- Atomic Mass Number
- The total number of protons and neutrons (designated by the letter A).
- Isotope
- Nuclei that contain the same number of protons but different numbers of neutrons.
The total mass of a stable nucleus is always less than the sum of the masses of its constituent particles.
- Mass Defect
- The difference in the mass of a nucleus and the sum of the masses of its constituent particles.
- Nuclear Binding Energy
- The amount of energy that must be put into a nucleus to break it into its constituent particles. It is the energy equivalent of the mass defect found by using E = mc2.
We can also look at a nucleus in terms of the forces that hold it together. The electric force described by Coulomb predicts that the nucleus should fly apart (since positive charges repel other positive charges). Another short-range attractive force must be acting within the nucleus.
- Strong Nuclear Force
- An attractive force that acts between all neucleons. Protons attract each other via the strong nuclear force while they repel each other via the electric force. The strong nuclear force is the strongest force, but it only acts over very short distances (less than 10-10 m).
Stable nuclei tend to have equal numbers of protons and neutrons for nuclei with Z = to about 30 or 40. If there are too many or too few neutrons relative to the number of protons, the nuclei tends to be unstable. For nuclei with Z greater than 30 or 40, stable nuclei have more neutrons than protons. There are no stable nuclei with Z greater than 82. They are all radioactive. For these very large nuclei, no number of neutrons can overcome the electric repulsion between protons.
In 1896, Henri Becquerel discovered that a photographic plate was darkened in the prescence of uranium. Marie
and Pierre Curie isolated two radioactive substances, polonium and radium. In 1899, Rutherford discovered that
uranium compounds produced three different kinds of radiation.
Types of particles involved in radioactive decay:
- Radioactive
- Nuclei that decay, emitting radiation, are considered to be radioactive. A new element is produced through this decay.
- Transmutation
- The changing of one element into another element via radioactive decay.
- Alpha Particle
- The particle emitted in alpha decay. It is essentially a helium
nucleus. It contains two protons and two neutrons. It has a charge of q=+2 and a mass of A=4.
When a nuclei decays by emitting an alpha particle, the number of protons is reduced by two and its mass is reduced
by four. Alpha particles are emitted by very large nuclei where the strong nuclear force is insufficient to hold the
nuclei together. It is abbreviated a or
 .
.
- Beta Particle
- The particle emitted in beta decay. Beta particles are
(negative) electrons emitted by the nucleus. It is not an orbital electron, but one created in the nucleus by
the decay of a neutron into a proton and an electron. It has a charge of q=-1 and essentially no mass. Beta particles
are emitted by nuclei that have too many neutrons relative to the number of protons. It is abbreviated b or
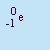 .
.
- Weak Nuclear Force
- Involved in the production of a beta particle in
the nucleus.
- Gamma Radiation
- Radiation emitted in gamma decay. Gamma radiation
is composed of high-energy photons. It is emitted by excited state nuclei. Gamma radiation has no charge and no
mass. It is abbreviated by g.
- Positron
- A positive electron produced in the nucleus by the decay of
a proton into a positron and a neutron. It has a charge of q=+1 and essentially no mass. They are emitted by
nuclei that have too few neutrons relative to their number of protons. It is abbreviated by
+b or
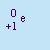 .
.
- Proton
- The nucleus of a hydrogen atom. The symbol is p or
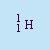 .
.
- Neutron
- The symbol is n or
 .
.
In all radioactive decay, the classical conservation laws hold. Energy, linear momentum, angular momentum, and electric charge are conserved. Also, the nucleon number is conserved. When balancing a nuclear reaction, mass and charge are conserved.
Rate of Decay:
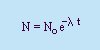
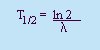
- Decay Constant, l
- A constant of
proportionality.
- Half-life (T1/2)
- The time it takes for half the original
amount of the substance (No) to decay.
Once, an atom was thought to be the smallest particle into which matter could be divided. Then, Rutherford found
that an atom was composed of protons and neutrons. The electron was discovered. Now, physicists believe
that particles out of which matter is composed can be grouped into two families, quarks and leptons.
- Quark
- Protons and neutrons are composed of quarks. A combination
of three quarks compose a proton. A different combination of three quarks compose a neutron. Particles that are
composed of three quarks are called baryons.
- Lepton
- Examples of leptons are electrons and neutrinos.
There are also particles that "carry" or transmit forces. The electromagnetic force is carried by photons. The
gravitational force is carried by gravitons (not yet detected). The strong nuclear force binds quarks into protons
and neutrons and is carried by gluons. The weak nuclear force is carried by three particles called bosons.
Each particle has an associated antiparticle. The positron is the electron's antiparticle. For every quark, there is
an antiquark. Particles composed of quark, antiquark pairs are called mesons.
Physicists are currently trying to create a Grand Unified Theory. In this theory, the four fundamental forces, gravitational,
strong nuclear, weak nuclear, and electromagnetic, were originally one force at the universe's beginning. The
electromagnetic and the weak nuclear forces were unified into the electroweak force in the 1970's.
Nuclear Energy
In 1934, Enrico Fermi and Emilio Segré bombarded uranium with neutrons, producing new radioactive isotopes.
In 1939, German scientists Otto Hahn and Fritz Strassmann found that barium was produced by bombarding
uranium with neutrons. Lisa Meitner and Otto Frisch proposed that the neutrons caused the uranium to divide
into two smaller nuclei, accompanied by a tremendous release of energy.
- Fission
- A division of a nucleus into two or more smaller daughter nuclei.
- Chain Reaction
- Neutrons produced by the fission of one nucleus
induce the fission of other nuclei.
- Fusion
- Two or more nuclei combine to form a larger nucleus. The sun produces its energy by nuclear fusion.
Nuclear Reactors
- Moderator slows down neutrons produced in chain reactions so they
can be more easily absorbed by other nuclie so fission continues.
- Control rods absorb neutrons, controlling the rate of reaction.
- Breeder reactors are ones in which some of the neutrons produced in the
fission of U-235 produce plutonium. This plutonium can be used in an atomic bomb.
Nuclear Physics Sample Problems
Nuclear Physics Homework
 .
.
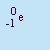 .
.
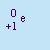 .
.
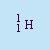 .
.
 .
.
 .
.
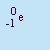 .
.
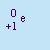 .
.
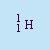 .
.
 .
.