Home Page of Peggy E. Schweiger
Thermal Energy & States of Matter
Kinetic theory of gasesparticles in a hot body have more kinetic energy than those in a cold body; as temperature
increases, kinetic energy increases. If the temperature of rises, the gas molecules move at greater speeds. If the volume remains the same, the hotter molecules would be expected to hit the walls of the container more frequently than the cooler ones, resulting in a rise in pressure.
Temperaturemeasure of an objectís kinetic energy; temperature measures how hot or how cold an object is with respect to a standard
Heat(symbol is Q; SI unit is Joule)amount of thermal energy transferred from one object to another due to temperature differences (we will learn in thermodynamics why heat flows from a hot to a cold body).
Q = m c DT
where m is mass in kg
c is specific heat of the material
DT = Tf - Ti in
°C
Mechanical Equivalent of HeatThe reversible conversion of heat energy and work. The calorie is defined as the amount of energy needed to raise the temperature of one gram of water at 14.5° one degree Celcius. The SI unit for work and energy is the Joule.
1 calorie = 4.184 J
Specific heat (c)a characteristic of a material; the amount of energy (measured in Joules) that must be added to raise the temperature of one kilogram of the material one degree Celcius or one Kelvin
specific of heat of water: c = 4180 J/kg K
specific heat of steam: c = 2020 J/kg K
specific heat of ice: c = 2060 J/kg K
Notice that the specific heat of water is very high - higher than ice and
steam. Water has a very high specific heat, meaning that it heats slowly
and cools slowly.
The specific heat of a material yields information about how the material heats and cools. If you add ten joules of heat to two materials, the one with the lowest specific heat will show the greatest temperature change. If you cool two materials ten degrees, the material with the greatest specific heat loses the most energy.
Energy transfer mechanisms:
- conduction (solids)-KE transfer due to collisions of particles
- convection (fluids)-KE transfer due to movements of fluids caused by
different densities at different temperatures
- radiation-energy transfer through a vacuum
Law of heat exchangethe sum of heat losses
and gains in a closed system is zero. When two bodies of unequal temperature are mixed, the cold body absorbs heat (raising its temperature) and the hot body loses heat (lowering its temperature) until an equilibrium temperature is reached. Thermal equilibrium exists when two objects that are in themal contact with one another no longer affect each other's temperature.
Qloss + Qgain = 0
Objects are in thermal equilibrium when they are at the same temperature.
Calorimeterdevice used to measure changes
in thermal energy
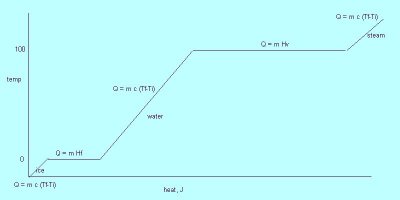
CHANGES OF STATE
The three most common states of matter are solid, liquid, and gas. When heat is added to a substance, one of two things can occur. The temperature can increase or the material can change to a different state. There is a fourth state of matter - plasma. A plasma is a state of matter in which atoms are stripped of their electrons. In a plasma, atoms are separated into their electrons and bare nuclei.
Let's look at ice (a solid) at a temperature of -5°. When heat is added to ice, its temperature increases until it reaches 0°. At this point, ice begins to melt--it changes its state from a solid to a liquid. The temperature remains constant at 0° until all the ice has melted. Now we have water at 0°. As heat is added to the water, its temperature increases until it reaches 100°. At this point, the water begins to boil, changing its state from liquid to gas. The temperature remains constant at 100° until all the water boils, turning into steam. Now we have steam at 100°. If you continue to add heat, the temperature of the steam begins to increase.
Heat of fusion (Hf)amount of energy needed to change 1 kg of a substance from a solid to a liquid. In physics, this is usually referred to as latent heat of fusion (Lf)
for water, Hf = 333,000 J/kg (333 x 103 J/kg)
Heat of vaporization (Hv)
amount of energy needed to change 1 kg of a substance from a liquid to a gas. In physics, this is usually called the latent heat of vaporization (Lv)
for water, Hv = 2,260,000 J/kg K (or 2.26 x 106 J/kg)
If energy is added to a system heating it and causing an increase in temperature, energy is positive; if energy is removed from a system cooling it and causing a decrease in temperature, energy is negative. If energy is added to a system causing a change in the state of matter from a solid to a liquid or from a liquid to a solid, that energy is positive. If energy is removed from a system causing a change in the state of matter from a gas to a liquid or from a liquid to a solid, that energy is negative.
At a phase change, the amount of heat given off or absorbed is found using:
Q = m H
where m is mass in kg and H is heat of transformation
No temperature change occurs at a phase change.
THERMAL EXPANSION
Most substances expand when heated and contract when cooled. The exception is water. The maximum density of water occurs at 4°. This explains why a lake freezes at the surface, and not from the bottom up.
A solid expands when heated and contracts when cooled: The length of a
material decreases as the temperature decreases; its length increases as
the temperature increases.
DL = a
L DT
where L is the length of the material
a is the coefficient of linear expansion
DT is the temperature change in °
C
A gas expands when heated and contracts when cooled: The volume of a gas decreases as the temperature decreases; its volume increases as the temperature increases.
DV = b
V DT
where V is the volume of the material
b is the coefficient of volume expansion
DT is the temperature change in °C
THERMODYNAMICS
Thermodynamicsstudy of properties of thermal energy
Internal energy(symbol is U; unit is J)sum of all the energy an object possesses; it cannot be measured; only changes in internal energy can be determined
1st law of thermodynamicstotal increase in the internal energy of a system is equal to the sum of the work done on the system or by the system and the heat added to or removed from the system. It is a restatement of the law of conservation of energy.
DU = Q - W
where Q is the net heat added to the system and W is the net work done by the system. In other words, heat added is positive; heat lost is negative. Work done on the system (an example would be compression of a gas) is negative; work done by the system (an example would be expansion of a gas) is positive.
changes in the internal energy of a system are caused by heat and work
2nd law of thermodynamics
- In natural processes, heat cannot flow from a cold to a hot substance
- Natural processes increase the entropy of the universe; with time, disorder cannot become order
- A heat engine cannot convert all its heat to mechanical energy. No machine is ever 100% efficient.
The second law of thermodynamics explains things that don't happen:
- Air molecules fill the room evenly, instead of all moving to one corner.
- A spoon reaches an equilibrium temperature, instead of one end being
cold and one end being hot.
- Coffee, swirling in your cup, will eventually stop swirling. The coffee doesn't spontaneously cool down and start to swirl around.
It is not possible to reach absolute zero (0 K). Since heat can only flow from a hot to a cold substance, in order to decrease the temperature of a substance, heat must be removed and transferred to a "heat sink" (something that is colder). Since there is no temperature less than absolute zero, there is no heat sink to use to remove heat to reach that temperature.
entropydisorder, chaos.
Heat engines:
- automobile engines-thermal energy from a high heat source is converted
into mechanical energy (work) and exhaust is expelled
- refrigerator-thermal energy is removed from a cold body
(work is required) and transferred to a hot body (the room. Another example is
a heat pump.
Drawing of a real engine showing transfer of heat from a high to a low termperature reservoir, performing work.

The purpose of an engine is to transform as much QH into work as possible. So...coffee can't
spontaneously start swirling around because heat would be withdrawn from the coffee and totally transformed
into work. A heat engine converts thermal energy into mechanical energy.
Drawing of a refrigerator showing transfer of heat from a low to a high temperature reservoir, requiring work.
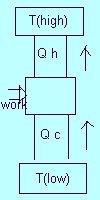 There is no perfect refrigerator because it is not possible for heat to flow from one body to another body at
a higher temperature with no other change taking place. The purpose of a heat pump
or a refrigerator is to convert mechanical energy into thermal energy.
There is no perfect refrigerator because it is not possible for heat to flow from one body to another body at
a higher temperature with no other change taking place. The purpose of a heat pump
or a refrigerator is to convert mechanical energy into thermal energy.
States of Matter Notes
Thermal Energy Sample Problems
States of Matter Sample Problems
Thermal Energy Homework
States of Matter Homework


