Carbon Info
Symbol: C
Atomic Mass: 12.0107 amu
Atomic Number: 6
Name Origin: from the Latin carbo (coal)
Discovered By: Unknown
Date of Discovery: Also unknown (a loooooong time ago)
Diagram:
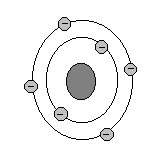
Placement on Periodic Table: second period, fourth group
Why it's there: It's in the fourth group because it has 4 valence electrons (which makes it stable).
Family: non-metal
Properties: Solid, black, melts at 3500.0 degrees C, boils at 4827.0 degrees C, hexagonal crystal structure, withstands most acids, gasses, corrosives, one of the better thermal conductors, resists electric current, 1 carbon can share electrons with up to 4 different atoms (4 valence electrons).
Isotopes: (5 isotopes) Carbon-11 has a half life of 20.3 min., C-12 is stable and is used as the base in atomic mass measurements (relative atomic mass), C-13 is stable, C-14 has a half life of 5730.0 years and is used in Carbon dating, C-15 has a half life of 2.5 seconds.
back to main page
