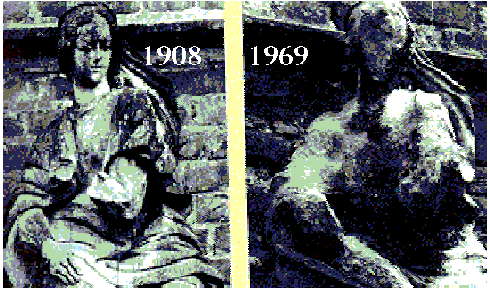
The effects of acid rain: left = before, right = after
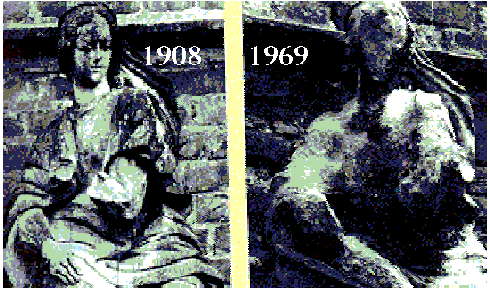
ABSTRACT
This paper reviews the way that acid deposition affects the environment as it disrupts the existing life. Acid deposition is defined before a thorough explanation of its formation is presented. An important part of understanding acid deposition comes from learning about its causes which the paper also discusses. Assessment methods for analyzing acid deposition in ecosystems are introduced in the text. From there, the paper describes the general effects that acidification has on our soils and waters. The paper also examines the many effects that pertain specifically to aquatic and forest ecosystems. Moving on, it cites particular cases about the depletion of soil aluminum and the lakes in Canada where changes have taken place because of acid deposition. At the conclusion of the paper, solutions are suggested for the situation.
INTRODUCTION
Acid deposition bombards the environment causing significant changes. Just ten years ago, little was known about this phenomenon commonly coined today as acid rain. Scientists first became aware of the possibility of acid deposition when they noticed subtle differences in the land and water, but at the time, it was a controversial issue to whether or not the cause was acid deposition. In actuality, this is not a recent occurence; as far back as the 1600's, scientists saw the awful effects that the pollution from industry was having on the surrounding vegetation. Acid deposition is defined as the process of depositing acidic pollutants from the earth's atmosphere to the earth's surface in both wet and dry forms. Acid precipitation, or "acid rain," only includes the wet forms of this pollution as it falls as rain, sleet, snow, fog, and cloud vapor (Gow and Pidwirny 1997). It contains a higher concentration of hydrogen ions than of hydroxl ions (McGraw 1992). Normal precipitation is slightly acidic with a pH between 5.0 and 5.6, but anything less than 5.6 is considered acidic (Gow and Pidwirny 1997). Thirty percent or more of acid deposition is in the dry form, but very little information is recorded about dry acid deposition (McGraw 1992).
FORMATION OF ACID DEPOSITION
Most formation of acid deposition results from human interference in the environment. Data suggests that humans cause 95% of the acid deposition that occurs on earth. Nitrogen oxide and sulfur oxide from industry and automobiles are major componets of acid deposition that become acidic as they react with water in the atmosphere (Gow and Pidwirny 1997). The most common strong acids in acid precipitation are sulfuric, nitric, hydrochloric, phosphoric, organic, acetic, and carbonic acids (Reuss and Johnson 1986). The problem with these acidic parts in the atmosphere is that they can be carried great distances from their initial site creating widespread effects. The major sources of sulfur oxide are coal burning, smelting of metal sulfide ores, volcanic eruptions, and organic decay. Similarly, the major sources responsible for nitrogen oxides are the combustion of oil, coal, and gas, soil bacterial action, forest fires, volcanic action, and lightning (Gow and Pidwirny 1997).
GENERAL EFFECTS
Three major effects in soil are cation depletion, aluminum mobilization, and pH depression (Reuss and Johnson 1986). The environment is being forced to adapt to these new changes. The harsh effects of this phenomena on the environment are rampant. Increased acidity leaches important nutrients like calcium, potassium, and magnesium from the soil and harms the vegetation. This, in turn, results in a decline in plant growth rates. Aluminum damages roots when it becomes more mobile in the soil as it reacts with the acid rain. Germination of seeds is inhibited with a reduction in pH. Decomposition and nutrient cycling also becomes inhibited as the soil organisms die. Besides affecting the soil that the plants live in, the acidic precipitation damages the foilage as it hits the leaves directly. Dry deposition can harm the plants ability to retain the water it needs to survive.
Just like plants, humans pay a price for the the changes that are occurring. Research has found that humans suffer from toxic metals like mercury and aluminum that are reaching drinking water, crops, and fish. Humans ingest these metals when they drink and eat the products. Scientists have discovered that aluminum is possibly linked to Alzheimer's disease. An increase in respiratory illnesses has also been noted in areas with substantial amounts of acid deposition polluting the atmosphere.
Acid deposition has begun to affect other objects like cars and buildings. The acid in the rainfall rusts the material (Gow and Pidwirny 1997). As acid deposition causes more harm, it becomes evident that it is no minor problem.
EFFECTS ON AQUATIC ECOSYSTEMS
The aquatic ecosystems found on earth are highly affected by acid deposition. As one would imagine, major shifts in species composition occur with the imposing changes of the increasing acidity on the species' environment. Acid-tolerant species increase in abundance, while the acid-sensitive species decline greatly in numbers. Most eventually become extinct because they cannot adapt to the new water chemistry (Charles 1991). As the acidity of the waters increases, the species richness decreases since only a fraction of the species present in aquatic ecosystems are able to survive and reproduce. A good demonstration of these effects is one lake, Lake 223 in Northwestern Ontario, which was gradually acidified over an eight year period (1977-1983) from a pH of 6.8 to a pH of 5.0 (Charles 1991). According to the results of this experimental acidification, many significant changes occurred within the ecosystem. Species composition was one noticeable change. For example, by the fifth year, scientists observed an increase in biomass and abundance of dinoflagellates and blue-green algae. The species composition had stabilized and the total biomass and productivity were nearly normal by the eighth year (Charles 1991). The zooplankton reacted similarly to the phytoplankton. Initially, they experienced a loss of one species, the Diaptomus sicilis, and a decline in another species, the copepod Epischura lacustris. By the end of the gradual acidification, these zooplankton also stabilized (Charles 1991). These are examples of acid-tolerant species because over a gradual period of time they were able to adapt to the new water chemistry and to survive the change in acid content.
Scientists found thick mats of filamentous algae appearing in the littoral areas of the lake. Algae increases its productivity with higher acidic contents. Unlike algae, invertebrates, like the crayfish, are harmed by acid deposition because it slows the process of the hardening of its exoskeleton. After recurring increases in acidity, the crayfish went to near extinction. By the end of the experiment, they had in fact gone extinct. Fish are acid-sensitive so they, too, did not react well to the changes in their ecosystem. From this evidence, we see that aquatic ecosystems cannot remain unchanged when acid deposition imposes on their environment (Charles 1991).
EFFECTS ON FOREST ECOSYSTEMS
Forest ecosystems provide an environment in which a large variety of organisms subside and it also contains several important nutrient cycles that occur simultaneously. Since it is a busy area in nature, the effects of acid deposition are profound. Three principal pathways of acid rain effects have been taken into account by scientists studying the effects of acid deposition on soil organisms and decomposition processes. Scientists examine the direct effects such as every aspect of proton toxicity that cause an immediate response of the organisms, indirect effects via modifications of the abiotic environment including aspects like the association of chemical stress with acidification and the alteration of the structural characteristics of the soil habitat, and indirect effects via alterations of the biotic environment like the modification of the predation process. In searching for any detail demonstrating these effects, they found data on disease incidence, soil and its organisms, and two nutrient cycles (Godbold and Huttermann 1994).
According to Godbold's book, Effects of Acid Rain on Forest Processes, there is an increased frequency of disease incidence in the forest due to acid deposition (Godbold and Hutterman 1994). Research has determined that acidity enhances abiotic diseases, foliage diseases, and root and stem decays. Frost damage, an abiotic disease that results from the freezing of tissues, increases with the amount of acid pollution in the atmosphere. Damage is much more severe in areas receiving high amounts of pollutants. This foliage disease causes infections in plants that are not readily detected; therefore, it characterizes a decline in a significant amount of forest life. Another example of a foliage disease is the pathogen, Anthracnose. It appeared in the eastern United States and created an epidemic where inoculations failed unless an acid mist treatment preceded the experiment. With higher acidity, more root damage exists. Also, a higher frequency of putative pathogens were found in acid soils (Godbold and Hutterman 1994).
The reaction of soil biota to acid deposition relies on the buffering capacity of the soil. In poorly buffered soils, acid rain accelerates the shift from base-rich to acid soils so that the soil can better tolerate acid. Humus disintegration is this initial process of transferring the state of the soil. The altered soil lacks nitrates. As acid hits the forest ground, it limits the thin litter layer on the surface. High acidity of acid soils kills the soil-burrowing animals, and bacterial activity is limited. Within the soils of the forest ecosystem, species diversity is lower, especially in bacteria and fungi. Bulk parameters, biomass and total microbial activity, are depressed by the increased input of protons into the environment (Godbold and Hutterman 1994).
Acid deposition causes alterations in the nutrient cycles and decomposition rates in forests. Strong accumulations of primary and secondary compounds of carbon have been detected. As a result of this change in the carbon mineralization rate, the composition and the state of the soil organic matter changes (96-99). The nitrogen mineralization rate, specifically nitrogen ammonification, also increases with acid soil. Although increases in these two rates are noted, the acid actually reduces the availability of carbon and nitrogen to microorganisms. Along the same line, the incorporation of nitrogen into the soil from dead organisms, nitrogen transfer to plants, and denitrification are all slowed down (Godbold and Hutterman 1994).
SPECIFIC CASES CITED
Depletion of soil aluminum by acid deposition: In 1989, Mulder, Van Breeman, and Eijck looked at the depletion of soil aluminum by acid deposition. First, they took samples from the Netherlands and New Hampshire. Then, the scientists added acid solutions to the samples. Every twenty four hours they shook the suspensions. Afterwards, the different samples were evaluated. Usually, acid rain in acid soils is neutralized by aluminum found in the soil. However, when acid rain is overabundant in an area, the rate of depletion increases. According to their experiment, Only a minor fraction of soil aluminum is readily dissolved (Mulder et al 1989). The other percentage of aluminum is the problem; it is depleted at a rapid pace, and if it is not controlled, it will result in reduced acid neutralization in the soils. Leaching is one way that this important mineral is leaving the soil. The study states that the current depletion rates of extractable aluminum are signicantly higher than the rates of aluminum transfer from silicate-bound to extractable aluminum (Mulder et al 1989). The study also found that the current aluminum mobilization rate from the uppermost layers are lowest where the soil organic aluminum content is lowest (Mulder et al 1989). Therefore, not only are the levels of aluminum in the soil decreased, but the mobilization of the mineral is slowed down. The significance of the experiment is that it shows that an increase in acid in the environment destroys the stability of aluminum levels in the soil which directly affects the richness of the soils and the productivity of the organisms.
Canadian Lakes: Canada is a prime example of the effects of repetitive acidic deposition on an ecosystem. Canada has a serious problem with acid deposition because it is located near a enormous number of industries whose emissions pollute the atmosphere (Air Quality Research Branch 1997). Over 300,000 lakes in eastern Canada are vulnerable to the effects of acid deposition and 14,000 of them have lost a large amount of fish because the lakes have become toxically acidified. There has been a decline economically in the fishing industry. The forests are also in danger because of the acidic rainfall. In humans, respiratory problems are connected to the strong atmospheric pollution (Gow and Pidwirny 1997). Canada had more acid deposition entering their area than they knew what to do with due to the tremendous amount of emissions coming from the combustion of fuels. Another area with similar conditions is the Adirondack Mountains. It is sensitive to acidity, but most are rapidly flushed draining systems. The only true difference in situations is that the Adirondacks are controlled by overlying glacial till and bedrock (Baker et al 1991). These two specific situations demonstrate how easily acid deposition alters the environment.
SOLUTIONS FOR ACID DEPOSITION
Many people in Canada and the United States realized that this problem could not go on ignored. In 1991, these two countries established the Air Quality Accord which controls the air pollution across the international boundaries. The aggreement states that emissions that cause acid deposition must be permanently capped in both countries. The maximum amounts are 13.3 tonnes in the United States and 3.2 tonnes in Canada (Gow and Pidwirny 1997). Although the act does not end all the acid deposition that exists, at least it reduces it. Another step that industries can take is to install scrubbers on the top of the smokestacks to filter the air before it is released into the atmosphere. Besides eliminating some of the emissions by controlling the source, a method to reduce acid deposition a process called liming helps alleviate the problem. Liming is a technique that involves adding quantities of hydrated lime to polluted waters to increase the alkanity and pH (Gow and Pidwirny 1997). Of course, these solutions help lead the problem in the right direction, but they do not work all the time.
CONCLUSION
Countless factors affect the ecosystems on earth. Acid is a major cause of the decline of aquatic and forest ecosystems. Through researching this problematic phenomena, I encountered statements like, "After more than two decades of intense research, however, our view of the causal relationship among 'recent forest decline,' acid rain induced alterations of soil conditions, and soil biota is still more than scattered" (Godbold and Huttermann 1997). Statements like this one told me that the scientific world needs to discover more ways to handle acid deposition. If people use the solutions available today and still search for others, then it seems possible that acid deposition can be reduced to a point where it will not endanger organisms in the future.
ACKNOWLEDGEMENTS
I would like to thank the library assistants in the Life Science Library. Without them, I would have been lost when I was searching for my resources.
WORKS CITED
1. Gow, T, Pidwirny, M. 7 November 1997. Acid Rain and Deposition. Okanagan. On-line. Okanagan University College Library. Internet. Available www: http://royal.okanagan.bc.ca/mpidwirn/internet/atmosphereandclimate/acidprecip.html
2. "Acid Rain." 1992. McGraw-Hill Encyclopedia of Science and Technology. V 1: 59-62.
3. Reuss, JO, Johnson, DW. 1986. Acid Deposition and the Acidification of Soils and Waters. New York: Springer-Verlag.
4. Charles, Donald F. 1991. Acidic Deposition and Aquatic Ecosystems. New York: Springer-Verlag.
5. Godbold, DL, Huttermann, A. 1994. Effects of Acid Rain on Forest Processes. New York: Wiley-Liss.
6. Mulder, J, van Breeman, N, Eijck, HC. 1989. "Depletion of Soil Aluminum by Acid Deposition and Implications for Acid Neutralization." Nature 337 Q1 N2: 247-249.
7. Air Quality Research Branch. Acid Deposition. 7 November 1997. Canada. On-Line. Internet. Available www: http://airquality.tor.ec.gc.ca/aqrb/9495acid.html
8. Baker, LA, Herlihy, AT, Kaufmann, PR, Eilers, JM. 1991. N5009: 1151 "Acidic Lakes and Streams in the United States: the Role of Acidic Deposition." Science 252.
More Info on Acid Deposition:
Progress Reports of 1992-1993
Acid Deposition in Northeast Asia