
MY RESEARCH ACTIVITIES ON SEMICONDUCTING POLYMERS AND MOLECULES

I am researching the optical and electronic properties of semiconducting polymers and molecules.
In everyday use, polymers (commonly known as plastics) are used as electrical insulators and can be engineered to have high tensile strengths, e.g. Kevlar.
Polymers consist of long chains of repeating sequences, in which the repeat unit is usually made up of carbon and hydrogen atoms.
In most insulating polymers, the carbon atoms are each bonded to four neighbouring atoms and the bonds are usually oriented in a tetrahedral arrangement.
However, in most organic semciondcutors, the carbon atoms are each bonded to three neighbouring atoms, with the bonds oriented in a trigonal planar arrangement. The three sigma-bonds per carbon atom form the structural backbone.
This leaves one p-electron per carbon atom. These can overlap to form a molecular pi-orbital.
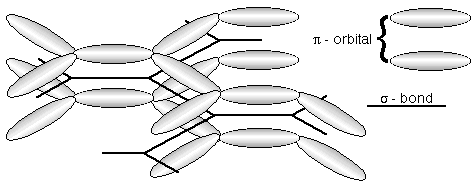
The molecular pi-orbital allows electrical charge to move relatively freely along the length of the molecule.
This delocalised bonding structure is usually drawn as an alternating sequence of single and double chemical bonds.
For this reason, the materials are called conjugated polymers and molecules. The structures of some typical conjugated polymers are shown below.

Because most molecules in living creatures are composed of carbon and hydrogen, these materials are also known as organic semiconductors. Their chemical structure is similar to that of naturally occurring dye molecules and photoreceptors, such as beta-carotene, the orange dye found in carrots and retinal, the purple photoreceptor within the human eye. The chemical structure of beta-carotene is shown below. Notice the similarity with polyacetylene.

These materials can be used in light-emitting displays, solar cells, transistors and diodes and when doped to a metallic form, as lightweight conductors for aircraft and electromagnetic screening.
The main advantage of these materials over conventional inorganic semiconductors, such as silicon, germanium or gallium arsenide is that conjugated polymers combine semiconductor properties with the processing advantages of polymers, so that it is relatively easy to prepare thin films of materials covering large areas,
e.g. for displays, by spin-coating, or dip-coating the substrates, without the need for expensive deposition equipment or the problems of matching crystalline lattice spacings between semiconductor layers.
Another important advantage is that the semiconducting properties can be adjusted by making chemical modifications to the basic polymer chain. Therefore, for example, it is possible to tune these materials to emit light in all colours from red to blue. (It is very difficult to produce blue light using inorganic semiconductors).
My Ph.D. research was concerned mainly with studying how charge is stored in these materials. Electric charge is not simply stored as free electrons and holes. Instead, there is a significant distortion of the chemical bond lengths (lattice relaxation) around an electrical charge.

The distortion of the chemical structure results in new molecular levels within the semiconductor energy gap and hence new optical absorptions, usually in the red to near infra-red region.

By making partially transparent MIS diodes (with a metal-insulator-semiconductors sandwich structure), it is possible to charge the materials by purely electrostatic means.
By applying an oscillating voltage to the gate electrode, the charge density within the device can be modulated, resulting in a tiny modulation of the absorption of the semiconductor film, (usually a change of about one-hundredth of 1% or less).
By studying the induced optical transitions under conditions of various charge densities and structural order, we can deduce that within these materials, charges can move around singly (known as polarons / radical cations or radical anions) or in pairs (doubly-charged spinless bipolarons or dications), and in partially crystalline systems, such as films of sexithiophene oligomers, we also find evidence for pi-dimers (inter-chain bipolarons).
During my reseach fellowship at St. John's College, I studied the response of polymer photodiodes built in a sandwich-structure, consiting of the polymer film between two semi-transparent metal electrodes. I compared the photocurrent action spectrum with various existing models for the spectral response of the photocurrent.
It was clear (also from the temperature-dependence of the photocurrent action spectrum and absorption spectrum) that the response is enhanced at the absorption edge. I considered correlations between chain segment length and the efficiency of inter-chain charge separation / transport.
During my post-doctoral research in Marburg, I am using electroabsorption and two-photon absorption to study the excited states of a ladder-type polyphenylene polymer with a high degree of intra-chain structural order.

MARK HARRISON'S RESEARCH PAGES


Mark Harrison mgh@who.net, Marburg, May 3, 1998











