|
RETINOPATHY & HYPOGLYCEMIA RISK Work, Beef UL DoesTable 1 Table 2 Table 3 Table 4 Table 5 Table 6 Table 7 Table 8 Figure 1 Figure 2 Figure 3 Figure 4 Implementation of intensive diabetes therapy for IDDMEveryone knows it requires brains to live long with diabetes, but to use insulin successfully requires more brains. Joslin. Gray, and Root (1) Note added in 1995 proof: Since this manuscript was written, the beef Ultralente insulin preparation has been discontinued. The announcement and publication of the results of the Diabetes Control and Complications Trial (DCCT) (2) and the Stockholm Diabetes Intervention Study (3) in 1993 resolved many decades of controversy. Future generations may very well question why it took us so long to make these fundamental observations. Perhaps the most important reason for this was that the tools to intensively treat IDDM were not available until the 1980s. However, the general philosophies for this therapy were actually introduced in the 1920s. E.P. Joslin was the first to suggest regular and systematic self-monitoring of diabetes control (glycosuria) before each injection of insulin (1). However, insulin preparations with prolonged durations of action were introduced in the 1930s and 1940s for added convenience (4). Although this therapy was generally able to avoid ketosis and hypoglycemia, some thought this actually represented deterioration in diabetes management (5). Certainly, reviews examining the natural history of the complications of IDDM since the discovery of insulin were quite discouraging (6,7). Whereas the history of insulin therapy for IDDM may be considered cyclical as in the 1980s when preprandial regular insulin again became fashionable, the achievement of normoglycemia or at least near normoglycemia would not be possible if not for self-monitoring of blood glucose (SMBG), first introduced in 1978 (810). Patients could, for the first time, be taught how to alter insulin administration based on glycemia. Alternatively, this information could be used to adjust food intake or the time between insulin administration and eating. Furthermore, diabetes management during illness became safer to manage at home. The other important tool introduced over a decade ago was the ability to objectively assess glycemic control with glycosylated hemoglobin levels (1113). From the University of Washington diabetes Care Center and School of Medicine, Division of Metabolism, Endocrinology, and Metabolism, Seattle, Washington. Address correspondence and reprint requests to Dr. Irl B. Hirsch, UWMC, 1959 Northeast Pacific Street. Box 356176. Seattle, WA 98195. ADA, American Diabetes Association: CSII, continuous subcutaneous insulin infusion: DCCT\. Diabetes Control and Complications Trial: IDDM, insulin-dependent diabetes mellitus: MDI, multiple daily injections: NIDDM, non-insulin-dependent diabetes mellitus: SMBG, self-monitoring of blood glucose: T50, time for 50% of 125I-labeled insulin activity to disappear: TAG, total available glucose. Side Bar - With the announcement of the Diabetes Control and Complications Trial, it is appropriate to review our current strategies for the treatment of insulin-dependent diabetes mellitus (IDDM). Since these strategies include an entire program of management, the term "intensive diabetes therapy" is used so that no one element of the program is emphasized. Not all patients should be expected to normalize glycemia: thus, individual patient goals are critical. All insulin preparations sold today have some antigenicity, although problems related to this are now rare. Although most patients with IDDM are given human insulin preparations first, there may be certain situations in which it is advantageous to use an animal insulin preparation for a particular pharmacokinetic advantage. There are a variety of pharmacokinetic issues that alter the absorption of insulin, and in general, regular insulin is absorbed more predictably than NPH or Lente insulin. Modern-day insulin regimens take advantage of the decreased variation in absorption of regular insulin with administration before meals. Patients need to be taught algorithms for insulin supplements and lag time (time between injection administration and eating) so that they may appropriately react to blood glucose levels out of their target range. There are a variety of newer options to manage the meal plan for patients using intensive diabetes therapy. The major risk of meticulous glycemic control is hypoglycemia, especially at night. There is also more evidence that rapid improvement of glycemic control may worsen preexisting diabetic retinopathy, and this deterioration may not be benign. Hopefully, further improvements in our technology will allow us to better manage IDDM more effectively and safety. This measurement allows both providers and patients to quantitatively review overall diabetes control and has been shown to be beneficial in motivating patients to better manage their diabetes (14). Despite these two important additions to help improve glycemic control, our attempts to mimic the normal B-cell are far from perfect. Exogenous insulin is administered into subcutaneous tissue in a massive bolus, as opposed to the normal pulsatile secretion into the portal circulation. Normally, hypoglycemia is prevented both by decreased insulin secretion in response to exercise and by appropriate release of glucagon and epinephrine once blood glucose levels decrease below a critical glycemic threshold. In patients with IDDM, any changes in insulinemia for exercise must be based on an educated guess and frequent SMBG to prevent hypoglycemia: glucagon and epinephrine secretions are often abnormal and may not protect from or respond to hypoglycemia (15,16). Some consider it surprising that we are able to maintain the usual level of metabolic control we do given these limitations (17). A more optimistic view is that the DCCT and the Stockholm Diabetes Intervention Study have conclusively shown that we can change the natural history of microvascular complications and that we should strive with our current technologies to maintain blood glucose levels as close to normal as possible in appropriate patients. Recent encouraging retrospective data shows that when a population of patients with IDDM was introduced to a diabetes management team, multiple injections, and an improvement in glycosylated hemoglobin levels, the cumulative incidence of diabetic nephropathy after 25 years of diabetes decreased from 30.0 to 8.9% (18). Taken together, our technology for diabetes therapy is imperfect and is related to serious side effects. The chronic nature of a condition that is affected by lifestyle changes makes it difficult for all patients to excel. Nevertheless, current therapeutic strategies should greatly improve the lives of many people with IDDM. In this review, the issues of importance to practitioners concerning intensive therapy of IDDM will be discussed. These include specific definitions, differences in insulin preparations and pharmacokinetic issues, treatment strategies, diabetes algorithms, and risks of therapy.
However, this definition focused primarily on the issues related to insulin and not on the other comprehensive components of a diabetes program. The terms intensive insulin therapy and intensive therapy have been used differently by different authors but are still most often used to focus on issues related to insulin therapy, namely, multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII) (2030). Others contend that intensive therapy comprises an entire program of diabetes management (17,3137) (Table 1). Although a multiple-component insulin program designed to provide physiological insulinemia is an important element of this program, this is only one aspect of the therapy, The term intensive insulin therapy is therefore not preferred since this tends to overemphasize this element of the program. Intensive diabetes therapy is more appropriate when referring to the modern-day management of IDDM. A multiple-component insulin program is one of the important elements of intensive diabetes therapy. Although this is most usually achieved with MDI or CSII, a program using only two daily injections may be effective for some patients (38). Due to limitations with this latter regimen, it is not commonly used for modern-day intensive diabetes regimens and indeed was not used in the intensive therapy group in the DCCT (2,32). Intensive diabetes therapy also requires that the patient carefully modify food intake, exercise, and insulin dose to obtain the desired glycemic targets. The meal plan needs to be flexible and adapted to the patients activity program (39). The use of frequent SMBG is critical for this type of program to succeed. SMBG allows patients to analyze responses to food, activity, and insulin (40). Patients and their health care providers may then use the results of SMBG to adjust any element of the therapy. This would include a predetermined set of algorithms for premeal insulin supplements (41). Certainly, regular SMBG should be considered routine for any patient with IDDM during acute illness.
Human UL Does NOT Human UL Does NOT Human UL Does NOT Work, Beef UL Does Table 1 Table 2 Table 3 Table 4 Table 5 Table 6 Table 7 Table 8 Figure 1 Figure 2 Figure 3 Figure 4 Another important element of intensive diabetes therapy is defined target blood glucose levels. The DCCT research group recommends that patients with IDDM maintain glycemic levels as close to the normal range as is safely possible (2). Similar recommendations were made by the American Diabetes Association (ADA) (42). Table 2 shows the blood glucose targets used for the DCCT. These glycemic targets would be for a young, otherwise healthy patient who recognizes symptoms of hypoglycemia, is motivated, and monitors blood glucose levels regularly. However, specific glycemic targets must be individualized, as circumstances may dictate altered glycemic targets. For example, the DCCT glycemic targets would not be appropriate for children younger than 2 years old, older patients with atherosclerosis, or patients with advanced complications (such as renal failure) (42). Patients who have hypoglycemia unawareness, who have significant psychiatric disease, or who are mentally retarded would also require glycemic targets different from those in Table 2. However, all of these groups may participate in an intensive diabetes therapy program as long as the actual targets are defined, The system of intensive diabetes therapy applies no matter what target is defined. Implementation of intensive diabetes therapy requires frequent contact between the patient and the diabetes management team (43). The benefits of a team approach have been well documented (44,45). Hopefully, one of the implications of the DCCT will be better acceptance of this team approach (46,47). It is also quite clear that patient education is essential for intensive diabetes management. In the DCCT, patients were initially hospitalized, generally for 24 days, to teach them the elements of MDI or CSII and to develop individual treatment algorithms (32). Patients and family members were also given an individualized diet prescription and instructions on the treatment of hypoglycemia and management of illness and ketosis, Thereafter, patients were seen weekly for further education until they were comfortable with the regimen. At this point, they were seen monthly for the remainder of the trial. Although this degree of interaction may seem excessive for most patients with IDDM, patient success would not have been possible without this extensive and continuing patient education. It is also clear that success depends on patient motivation. Even when all the elements of an intensive diabetes program are in place, treatment in some patients will not be successful. This was true in the DCCT (2) and in other populations (3,18). The responsibility for implementing and maintaining intensive diabetes therapy puts some degree of stress on each patient. The exact relationship between stress and diabetes is quite complex (48), although it appears that stress has an important impact on glycemic control through its impact on patient behaviors (49). Psychological support from a mental health specialist should be considered to help implement intensive diabetes therapy (50). Referrals would also be appropriate in patients with repeated episodes of severe hypoglycemia or diabetic ketoacidosis without an obvious cause, or who are considered "frustrating" (51). Women with IDDM appear to have a higher prevalence of eating disorders, and not surprisingly, many patients with IDDM with advanced complications have a high prevalence of depression and anxiety (51). It is therefore imperative to recognize these problems and treat them appropriately for successful implementation of intensive diabetes therapy. The other critical component of intensive diabetes therapy is an independent glycemic assessment with measurement of glycosylated hemoglobin levels (11 14). Whereas SMBG is used for daily assessment to guide treatment decisions for insulin adjustment and caloric intake, glycosylated hemoglobin levels are used as an overall report of glycemic control, The regular use of glycosylated hemoglobin levels has been shown to be beneficial to patients in helping them improve glycemic control (14). The ADA recommends that patients with IDDM have their glycosylated hemoglobin levels measured quarterly (52). However, a review of physician practice patterns show that this test is underutilized (5355). In summary, intensive diabetes therapy should be considered a comprehensive program of diabetes management. Due to the many complexities in the treatment of IDDM, several health care professionals are required for routine treatment. The goals of therapy and the tools used to achieve these goals will need to be individualized for each patient. Although insulin preparations are traditionally classified according to their time course of action, insulin species and degree of purity are also important influences on absorption characteristics. In general, the human insulin preparations have a shorter duration of action than their animal insulin counterparts (56) (Table 3). In certain situations, the use of a longer-acting animal species insulin may be advantageous.
RETINOPATHY & HYPOGLYCEMIA RISK Human UL Does NOT Work, Beef UL Does Table 1 Table 2 Table 3 Table 4 Table 5 Table 6 Table 7 Table 8 Figure 1 Figure 2 Figure 3 Figure 4 Before 1970, the insulin formulations available for clinical use were purified by recrystallization and contained significant amounts of impurities, including proinsulin, glucagon, somatostatin, pancreatic polypeptide, and vasoactive intestinal polypeptide (4). Standard insulin preparations contained 10,00020,000 ppm proinsulin (56). Improvements in purification techniques have resulted in an improvement in the animal insulin preparations commercially avail able today. Standard insulin preparations sold in the U.S. currently have only 1020 ppm of proinsulin while purified insulin preparations have <10 ppm (57). These improvements have resulted in a marked decrease in the problems attributed to immunogenicity of animal insulin preparations, such as insulin allergy, insulin resistance, and localized lipoatrophy (58). All insulin preparations sold today, including human insulins have some antigenicity (59). In general, beef insulin is more antigenic than pork insulin, which is more antigenic than human insulin (59,61). This increased antigenicity does not necessarily cause a deterioration of glycemic control (61). Further more, problems related to immunogenicity have been relatively rare in comparison to those seen when commercial insulin preparations were less purified (62). Theoretically, an unpredictable dissociation of an insulin-antibody complex could result in hypoglycemia. However, no relationship was shown between insulin antibody levels and severe hypoglycemia or metabolic control in a large group of patients with IDDM (63), and other studies have failed to demonstrate a relationship between glycemic control and insulin antibodies (59,6467).
Still, moderate to high antibody levels may be of clinical importance in some patients (6871). Most authors recommend starting new patients with IDDM on human insulin, although there does not appear to be any advantage to switching patients with otherwise stable disease to human insulin from an animal preparation (24,29,56,72,73 ). PHARMACOKINETIC ISSUES Insulin Absorption A goal of any insulin regimen is to achieve as predictable insulin absorption as possible. Unfortunately, erratic insulin absorption resulting in unexplainable levels of glycemia is common in IDDM, although it may be less of a problem in patients with non-insulin-dependent diabetes mellitus (NIDDM) (74). A major frustration for both providers and patients is that the day-to-day intra-individual variation in the time required to absorb 50% of the injected dose of insulin is ~25% and between patients is up to 50% (7577). However, when other end points are considered, such as the peak insulin concentration, the time to peak, or the area under the curve for insulin, the overall range of variation has been reported to be 0% to >100% (78). It is there fore not surprising that unexplained hyperglycemia or hypoglycemia is common for patients with IDDM (Table 4). Since this variability is similar for all insulin preparations, in absolute terms there will be less variability in the absorption of regular insulin (7679), with more erratic absorption with longer-acting insulin preparations (NPH and Lente [76,77] and human Ultralente [80]).
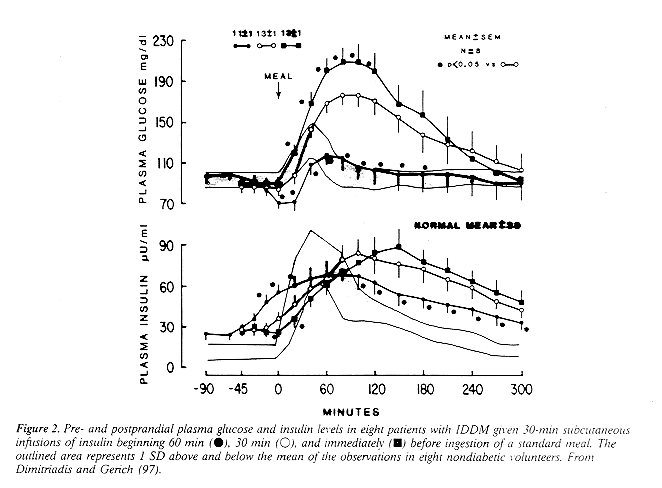
RETINOPATHY & HYPOGLYCEMIA RISK Human UL Does NOT Work, Beef UL Does Figure 1 Figure 2 Figure 3 Figure 4 Injection Sites Subcutaneous insulin is absorbed at different rates from different anatomical regions. These variations have been attributed to differences in blood flow (76,82). Absorption is most rapid from the abdomen, followed by the arms, with the buttocks and thighs exhibiting slower rates of absorption (7579, 8289) (Table 5). Intramuscular insulin injection (accidental or intentional) results in a more rapid absorption of insulin (89-97) The prolonged absorption of insulin when injected into the thigh has recently been re ported to result in a greater risk of nocturnal hypo g1ycemia with an MDI regimen (89). Since variations in absorption are sufficiently great to have an impact on glycemic control, the current recommendation is to use a single anatomic region for insulin injections, as opposed to the older teaching of site rotation (92) (Fig. 1). It has also recently been reported that there are consistent absorption differences within the same anatomic region. Insulin appears to be absorbed quicker from sites above the umbilicus than from sites below or lateral to the umbilicus (93,94). Therefore, any given injection (e.g. prebreakfast) should be administered in the same region to decrease day-to day variability. Insulin may also be absorbed more rapidly from an extremity that is exercised due to an increase in local blood flow (85,95,96). It would therefore be appropriate to inject the insulin into the abdomen before fore strenuous activity even if that injection was usually administered into the extremity being exercised, for patients participating in regular exercise programs, abdominal injections are the preferred site of insulin administration. Timing of Premeal Insulin The length of time between premeal regular insulin administration and food consumption (also called the lag time [41]) is perhaps the most underemphasized aspect of intensive diabetes therapy programs (Fig. 2). In general, the regular insulin needs to be given ~30 min before eating to ensure insulin availability during food consumption (86,9799), although with the faster action of human regular insulin, shorter lag times may he appropriate (30). The lag time can be altered depending upon the level of premeal glycemia. Thus, when blood glucose levels are above the target range, it may be desirable to increase the lag time. Alternatively, lag time should be decreased for premeal glycemia below the target level, and insulin should be administered just before eating for premeal hypoglycemia. One common reason for blood glucose fluctuations are frequent alterations in the lag time. Mixing Types of Insulin NPH (neutral Protamine Hagedorn) or isophane insulin is a mixture of Protamine, zinc, and insulin combined under precise conditions (100). In mixtures of NPH and regular insulin, the regular insulin retains its potency in a stable fashion for prolonged periods (101). All of the preparations of NPH insulin in have similar levels of Protamine, and it appears that there is a weak binding affinity for regular insulin in vitro. This absorption occurs within 20 min and appears to be ratio-dependent the greater the proportion of NPH, the greater the binding of regular insulin (102), However, in vivo this binding does not appear to be of any therapeutic significance (36). It is therefore permissible to mix NPH and regular insulin in the same syringe at any ratio needed for clinical purposes as the action profiles of the insulin will be maintained (75,83,103106). 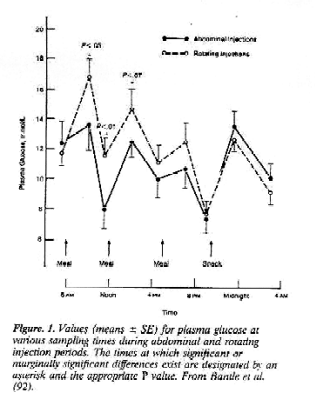
RETINOPATHY & HYPOGLYCEMIA RISK Human UL Does NOT Work, Beef UL Does Table 1 Table 2 Table 3 Table 4 Table 5 Table 6 Table 7 Table 8 Figure 1 Figure 2 Figure 3 Figure 4 The clinical effects of mixing regular insulin with an insulin from the Lente series are more problematic. Lente insulins contain excess zinc (107). When regular insulin is mixed with Lente or Ultralente insulin, binding will occur with the zinc, resulting in the regular insulin precipitating out of solution. This results in a blunting of the action of the regular insulin if the regular-Lente insulin mixture remains in the syringe for more than a few minutes (75,105. 106.108110). With beef-pork insulin, this process begins 15 to 20 min after mixing and progresses for up to 24 h (103). This process is even more rapid with human insulin (106). The activity of the regular insulin is maintained if mixing is accomplished in a syringe immediately before injection. Therefore, this issue is not a contraindication for an insulin program using Lente or Ultralente insulin. The only phosphate-buffered regular insulin currently available in the U.S. is Velosulin (Novo Nordisk, Princeton, NJ). Velosulin is incompatible with Lente series insulins because the phosphate precipitates the zinc from suspension and insulin activity will be lost. Human insulins (except Velosulin with Lente insulins) can also be mixed with other species of insulin (29).
RETINOPATHY & HYPOGLYCEMIA RISK Work, Beef UL DoesTable 1 Table 2 Table 3 Table 4 Table 5 Table 6 Table 7 Table 8 Figure 1 Figure 2 Figure 3 Figure 4
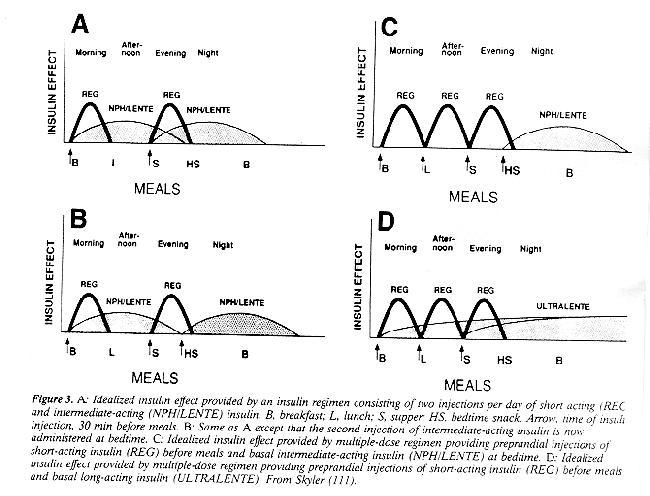
RETINOPATHY & HYPOGLYCEMIA RISK Human UL Does NOT Work, Beef UL Does Table 1 Table 2 Table 3 Table 4 Table 5 Table 6 Table 7 Table 8 Figure 1 Figure 2 Figure 3 Figure 4 Pubertal patients usually require 1.01.5 U kg (73,113) due to physiological resistance to insulin (114,115). Insulin requirements decrease after puberty, but it is common to encounter high school and college-aged patients who are over insulinized due to loss of medical follow-up from their pediatricians. Other factors that will increase insulin requirements include intercurrent illness (including surgery [116]), pregnancy (117), and concomitant use of glucocorticoids (118). Single-Dose Regimens Once-daily insulin injections first became popular in the 1940s and 1950s and by the 1960s had become standard therapy in many clinics, mostly because of convenience (4,19,56). A single injection of long-acting or intermediate-acting insulin, with or without regular insulin, does not mimic the normal pattern of insulin release (119). Thus, even patients not striving for meticulous glycemic control have difficulty with this regimen due to lack of flexibility with meal times. The exception to this are patients still making significant endogenous insulin, soon after diagnosis (12). Nevertheless, many physicians continue to routinely prescribe this therapy for all of their patients with IDDM (54). Twice-Daily Regimens NPH (or Lente) and regular insulin administered before breakfast and supper, the so-called split and mixed insulin regimen (19,111,121,122), has been perhaps the most popular insulin regimen during past few decades (Fig. 3). This regimen provides insulin availability for each meal and for most patients, sustained insulin availability overnight. The major advantage to this regimen is that requires only two injections. Also, algorithms favor regular insulin before breakfast and supper may incorporated when requirements change. The disadvantages of this regimen will often preclude patients from using this program. The first difficulty relates to time-action profile of the NPH or Lente insulin preparations. As noted from Table 3, the intermediate-acting human insulin preparations have an onset of action ~24 h after injection and produce peak insulin levels ~48 h after injection (with significant variation as noted above). Therefore, when intermediate-acting insulin is ad ministered before supper, it often does not sustain its effect through the night, and fasting hyperglycemia results. High morning blood glucose levels are exacerbated by the dawn phenomenon, a time of relative insulin resistance (123125). However, the extent to which the dawn phenomenon contributes to early morning glycemia is controversial (20). Nevertheless, attempts to correct the fasting hyperglycemia by increasing the dose of presupper NPH or Lente insulin results in greater nocturnal hyperinsulinemia, during which time insulin requirements are at their lowest (126). The other disadvantage with this regimen is that flexibility in mealtimes is greatly limited. If lunch is not eaten on time, peaking NPH or Lente insulin from the morning may result in hypoglycemia if blood glucose levels were well-controlled earlier. Delaying supper without additional insulin will result in hyperglycemia due to dissipation of the morning insulin. Because of the above problems, twice-daily insulin injections were used only in the conventional therapy group of the DCCT (32). Glycemic control with this regimen has not been shown to differ from once daily injections (127). Except for patients producing some endogenous insulin and maintaining very regular life styles, it is unlikely that patients using this regimen will be able to achieve normal or near-normal levels of glycosylated hemoglobin (30). Nevertheless, this regimen may still be appropriate for those patients with limited physical and intellectual capabilities or those patients for whom meticulous glycemic control is not appropriate. MDI: Split and Mixed Program with Bedtime Intermediate-Acting Insulin One popular solution to the problem of nocturnal insulin replacement noted above is to delay administration of the intermediate-acting insulin until bed time (19,128) (Fig. 3). Besides providing higher prebreakfast serum insulin levels which better match insulin requirements, nocturnal hypoglycemia should theoretically be less of a problem. Another advantage is that it is quite simple to switch from the twice-daily regimen since the dosing will be similar, although the morning regular insulin may need to be decreased since fasting and postbreakfast blood glucose levels are improved (128). Mealtime flexibility is not improved with this regimen, and many patients are not willing to administer three daily injections. The other problem with either of the split and mixed programs is related to the morning intermediate-acting insulin. Since NPH and Lente insulin preparations have relatively broad peaks, optimal insulin availability cannot be provided for lunch. Surprisingly, it was also shown that as afternoon blood glucose control is mainly dependent on morning regular insulin rather than morning intermediate-acting insulin (129). Thus, altering the morning NPH or Lente insulin to correct afternoon blood glucose levels may not be appropriate. MDI: Premeal Regular and Bedtime Intermediate-Acting Insulin The problem of midday glycemia can be improved by reducing or even eliminating the morning dose of NPH or Lente insulin and adding an injection of regular insulin before lunch (Fig. 3). This regimen uses three preprandial injections of regular insulin and an injection of intermediate-acting insulin at bedtime (19,23). As before, regular insulin can be adjusted based on premeal glycemia and anticipated carbohydrate intake. The disadvantage of this regimen is that long intervals between meals cannot occur without metabolic compromise as the regular insulin administered previously will dissipate (130). A small dose of prebreakfast NPH or Lente insulin will help smooth out daytime glycemia somewhat acting as a basal insulin, but there can still be problems with peaking intermediate-acting insulin resulting in the necessity to eat lunch at a fixed time or else risk hypoglycemia. The use of the convenient insulin pen devices is another advantage to an MDI regimen such as this (131136). There is no consensus on whether the use of the pens per se improves glycemic control, or if studies examining this issue caused patients to pay increased attention to their diabetes (137). However, it appears that absorption of regular insulin from an insulin pen device is slightly faster than a conventional injection (138). Patients also appear to prefer the pen injector compared with a syringe (139,140) or even an insulin pump (141). Some patients may find the pens so convenient that they believe they can eat when they want to. Without basal insulin, this behavior can result in ketoacidosis (142).MDI: Premeal Regular with Basal Ultralente Insulin Although
the MDI regimen with premeal regular insulin and
bedtime intermediate-acting insulin has become
the most widely used insulin program in some
parts of the world, there may be less blood
glucose fluctuations when basal insulinemia is
provided with Ultralente insulin (Fig. 3). A
basal insulinemia with Ultralente
insulin allows patients more flexibility with
mealtimes since if insulin dosing is correct,
metabolic control should not deteriorate if a
meal is missed.
Data
are means ± SE (range). From Hildebrandt et al
(150). Table 6
T50 for the species of
insulin, dose, and region indicated Abdomen Thigh 6 U 6 U 24 U Human
Ultralente 9.4 ± 1.4
(4.1-15.6) NS 13.0 ± 2.1
(6.7-21.3) NS 15.1 ± 2.5
(5.8-24.8) - P < 0.01 NS Beef
Ultralente 16.1 ± 1.4
(4.1-15.6) NS 21.4 ± 4.8
(8.8-49.6) NS 44.1 ± 8.6
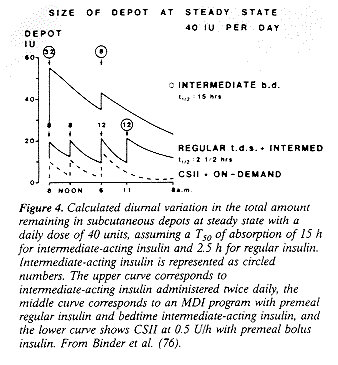
(18.3-91.0) RETINOPATHY & HYPOGLYCEMIA RISK Human UL Does NOT Work, Beef UL Does Table 1 Table 2 Table 3 Table 4 Table 5 Table 6 Table 7 Table 8 Figure 1 Figure 2 Figure 3 Figure 4 As seen in Table 3, the human Ultralente insulin has a broad peak at ~216 h with a duration of action of at least 24 h (75,143). Therefore, human Ultralente insulin is best used as a twice-daily preparation (34) or, if administered once daily, at bedtime (144). Human Ultralente insulin administered once daily in the morning will result in fasting hyperglycemia (145), although this will not necessarily affect glycosylated hemoglobin levels (146). Due to the prolonged peak and duration of the human Ultralente compared with intermediate-acting insulin preparations, presuppertime human Ultralente insulin will result in improved fasting blood glucose levels compared with NPH (147,148) or Lente (149) insulin administered at the same time. This may be especially beneficial for those patients who refuse to inject their insulin at bedtime. However, nocturnal hypoglycemia may be a greater problem with human Ultralente insulin administered before supper (149). Although several reports have suggested that human Ultralente insulin may act as a suitable basal insulin (143,150), others have not (80,151). Freeman et al. (80) showed the onset of action of the human ultra Lente insulin to begin after 24 h, with broad peaks (occurring between 6 and 12 h (80). Both these authors and another group (152) concluded that human Ultralente insulin did not result in basal insulinemia and mimics the pharmacokinetics of NPH or Lente insulin preparations. Fisken and Goulbourn (151) showed in problems of "unheralded, severe hypoglycemia" when used as a basal insulin (151). Ultralente insulin was initially developed to produce basal insulinemia (15), and it is clear that this is not the case with the human Ultralente preparation for many patients. On the other hand, beef Ultralente insulin appears to act as a true basal insulin. For example, Rizza et al. (154) found that plasma free insulin concentrations remained relatively stable in six patients with IDDM during a 40-h period in which no regular insulin was administered. Due to the long half-life and lack of any peak of beef Ultralente insulin, these investigators also concluded that a single daily injection of beef Ultralente should be adequate to maintain basal insulinemia in most patients. Although there is a theoretical concern about prolonged and severe hypoglycemia from beef ultra Lente insulin due to an overlapping interaction between the metabolic activity of the insulin of the current injection and that of the previous day (155), there have not been any reports of this problem. Similar to other insulin preparations, the absorption characteristics of beef Ultralente will depend on a variety of factors, such as injection site and size of dose. For example, differences in the calculated time for 50% of 125I-labeled insulin activity to disappear (T50) have been shown to be quite dramatic for both human Ultralente and beef Ultralente insulin when injection site and size of dose are considered (Table 6) (150). As can be appreciated, absorption is generally more rapid for human Ultralente insulin. Although the calculated mean T50 was not different between the low-dose and high-dose insulin for either type of insulin, the beef Ultralente injected into the thigh compared with human Ultralente showed higher residual activities with the 24-U dose after 12 h (150). Perhaps more impressive from these data are the dramatic ranges in the absorption characteristics from this group of eight subjects. Taken together, when one attempts to achieve basal insulinemia in an MDI program, the use of beef Ultralente insulin may be considered as the basal component. Others prefer the use of twice-daily human Ultralente insulin, at least for patients not previously taking animal insulin (17). The major argument for not using beef Ultralente relates to the increased antigenicity of animal insulin preparations. However, as noted above, this generally has no clinical significance with the standard insulin preparations sold today. In theory, it seems possible to administer the beef Ultralente insulin once daily. Because of variations that can occur with insulin absorption, we ask patients to mix their beef Ultralente with their premeal regular insulin before breakfast and supper. By administering the beef Ultralente insulin twice daily, any variation in absorption from a single injection should be minimized. The efficacy of the beef Ultralente insulin can be assessed best with measurement of the fasting blood glucose level. Alternatively, patients could skip lunch to assess basal daytime insulinemia, but most patients are not willing to do this. Patients may begin with 4045% of their total daily insulin dose with beef Ultralente, divided up equally before breakfast and supper. Most patients will require about 50% of their total as beef Ultralente, but there is considerable variation in this. Regular insulin is administered before each meal. It must be emphasized to the patient that due to the long duration of action of the beef Ultralente it will require at least 34 days before any type of decision regarding the beef Ultralente dosing can be made. Some authors have recommended a loading dose of the beef Ultralente insulin due to its long duration of action (154), but it is quite safe to supplement this with added regular insulin during the first 2 days after switching from another regimen. The majority of our patients with IDDM are quite satisfied with a beef Ultralente/regular insulin regimen. Patients (especially adolescents) who are unwilling to give a prelunchtime injection do better with another program. Human Ultralente insulin may be used in those patients who are fast absorbers of NPH or Lente insulin and are not interested in a basal/bolus regimen. For these patients in particular, who tend to be at or below ideal body weight, human Ultralente insulin should not be considered as a basal insulin. CSII Since only regular insulin is used, perhaps the most precise way to approximate normal insulin secretion is to use an insulin pump in a CSII program (19,29, 30,156,157). Subcutaneous insulin is continuously delivered by the pump in microliter amounts, mimicking basal insulin secretion. Both of the available pumps in the U.S. can be programmed for multiple basal rates, allowing a mechanism to help prevent nocturnal hypoglycemia or counteract the dawn phenomenon (20,158). Although unique patterns of basal infusions may be required in patients requiring them (159), the majority of patients do quite well with one to three different basal rates. In addition, since there is a lag of about 3 h between the time basal insulin rate is changed and the time insulin absorption rate changes (160), there are few theoretical reasons to have the delivery improves (such as with implantable pumps [161]), there maybe a greater need for more basal rates. Bolus insulin is administered before meals (as with an MDI program) to provide sufficient insulin for anticipation of the carbohydrate intake. There fore, if insulin dosing is correct, it should be possible to omit, delay, or alter meal size without any compromise in glycemia (162164). The syringe reservoir and infusion set are usually changed every 13 days. There has recently been some controversy about which type of regular insulin may be used. From a 6-month crossover study with 28 patients in 1985, Mecklenburg and Guinn (165) reported that 90% of insulin occlusions occurred with an unbuffered beef-pork insulin, while 10% of these episodes were with a buffered pork insulin. A later study confirmed a significant decrease in insulin obstruction with buffered purified pork insulin compared with nonbuffered purified pork insulin (166). As noted above, the only phosphate-buffered insulin available in the U.S. today is Velosulin human. Since then, there have been anecdotal reports that there have not been excessive problems with occlusions with either of the two human regular insulin preparations available in the U.S. Humulin R (Lilly, Indianapolis, IN) and Novolin R (Novo Nordisk) (L.P. Fredrickson, personal communication). The mechanism of the occlusion is unclear but may be the result of a fall in pH toward the insulin isoelectric point due to CO2 permeability in the tubing (167). It is possible that improvements in the insulin tubing have also contributed to the decrease in this problem. The current tubing, composed of polyolefin, may result in fewer occlusions than the older polyvinylchloride tubing. In any event, although a significant number of patients are using nonbuffered insulin preparations for their pumps, only buffered insulin should be recommended until this issue is studied again. There has been much written about the potential pharmacokinetic advantages of CSII. Because only regular insulin is used, one would expect more predictable insulin absorption since the variation in absorption of insulin is least with short-acting insulin and greatest with longer-acting insulin (76). This factor is particularly important in patients receiving very small doses of insulin. The lack or subcutaneous depot is another potential advantage to CSII (76,79,156,160). Disadvantages of large depots of insulin include unpredictable mobilization by in creased blood flow due to exercise (76,95), sauna (168), or massage (169). As can be seen from Fig. 4, regimens with a larger proportion of intermediate-acting insulin will create the greatest subcutaneous depot (76).
CSII may be used for patients with hypoglycemia unawareness and gastroparesis (170). The more predictable absorption of regular insulin with an increase in glycemic targets allows for safer diabetes management in this challenging patient population. Hypoglycemia unawareness should be considered an important indication for CSII, contrary to older recommendations (171). Studies comparing glycemic control in patients using CSII and MDI have shown either no difference or an improvement in glycosylated hemoglobin levels with CSII (37,130,172180). However, it is difficult to draw any conclusions about this since study populations, study durations, and patient and provider experience with CSII were different. Currently, CSII is used infrequently by patients with IDDM in the U.S. Perhaps the most important reason for this is that it increases the workload for both the physician and the patient, especially for physicians who do not have access to a nurse educator with experience in CSII (156,157). Added expense is another problem. A new pump now costs approximately $3,800, and pump supplies may cost an additional $100200 each month. Patients need to be selected carefully before pump therapy is begun. It has been shown that younger patients, who were more likely to be women and who had a shorter duration of diabetes, were more likely to discontinue CSII therapy (181). It was recently reported that 9 of 49 CSII users discontinued treatment after 5 months (182). Three of these patients showed a deterioration of glycemic control, four were unable to accept the pump, and the other two patients were noncompliant with SMBG and monitoring of urinary ketones. It is also interesting that the patients who discontinued CSII tended to be blue-collar workers (182). In practice, therapy may be initiated with a basal dose not to exceed 4050% of the patients total daily requirement. Since insulin requirements are often decreased with CSII, conservative estimates are appropriate and total dose should be decreased by about 10%. Bolus insulin needs depend on eating habits, but at our center, we typically begin with 20% of the daily dose before breakfast. 15% before lunch, and 15% before supper. Small doses may be required before the bedtime snack, but this is often omitted when CSII is initiated (157). DIABETES ALGORITHMS An intensive diabetes program cannot be successful unless the patient learns how to take the appropriate action in response to blood glucose or an unusual situation that will affect glycemia. These algorithms for diabetes management take into account insulin dose, lag time, carbohydrate intake, and activity. Patient education needs to include an action plan so that all of these components can be altered as the situation dictates. Algorithms for insulin dose changes should not be confused with sliding scale insulin (183,184), which pertains to the retrospective correction of hyperglycemia with regular insulin with out regard to caloric intake or physiological insulin delivery. An insulin supplement is defined as a temporary dose of regular insulin administered to prevent or correct a blood glucose level outside of the target range. A supplement may be added if premeal hyperglycemia is present, if an unusually large meal is anticipated, or if it is known that usual physical activity will not be performed. It is critical that lag times be incorporated with these supplements. Supplements administered to correct hyperglycemia, e.g., after a larger than anticipated meal or during an acute illness, need to be used with caution for insulin regimens using NPH, Lente, or human Ultralente insulin preparations. In addition, regular insulin (or bolus insulin, in the case of ) should be used with caution at bedtime due to the decrease in insulin requirements in the early morning (20). The continuing need for compensatory insulin supplements due to a pattern of unexplained blood glucose levels above the target range (e.g., continued fasting hyperglycemia) indicates that an adjustment should be made for the relevant insulin component. Adjustments then, are modifications in the basic insulin dose made in response to a pattern of glycemia over several days, assuming there are no unusual circumstances causing the blood glucose levels to be outside of the targets. Adjustments may apply to any type of insulin.
RETINOPATHY & HYPOGLYCEMIA RISK Human UL Does NOT Work, Beef UL Does Table 1 Table 2 Table 3 Table 4 Table 5 Table 6 Table 7 Table 8 Figure 1 Figure 2 Figure 3 Figure 4 Table 7 represents a sample algorithm for premeal regular insulin used with an Ultralente/regular or CSII regimen. This should be considered a starting point since patients often require individual algorithms. This example can also be used with other MDI programs, although regular insulin supplements may need to be changed if an intermediate-acting insulin preparation is used. Since carbohydrate content in a meal is quantitatively more important than protein or fat content in determining insulin requirements (185), there has been greater attention placed on carbohydrate counting as a way to more precisely determine premeal regular insulin doses (186189). Five of the DCCT clinics used carbohydrate counting as the nutrition intervention (186). With this method, patients are taught how to count how many grams of carbohydrates they anticipate eating, and premeal regular insulin is calculated based on a ratio of insulin to carbohydrate content. This ratio needs to be individualized and will vary between patients due to body weight (Table 8) and different levels of insulin sensitivity and within patients based on variations in activity levels and time of day. Therefore, insulin algorithms as exemplified in Table 6 can be used to supplement a premeal dose of regular insulin derived from carbohydrate counting. Alternatively, the traditional exchange system (190) or total available glucose (TAG) (191) meal-planning strategy may be used. With the TAG approach, 100% of carbohydrate, 58% of animal protein, and 10% of fat will be available as glucose for cellular use. All meals and snacks are then given a TAG allotment, and this may work quite well in conjunction with the exchange system. For example, one fruit exchange would contribute 15 g TAG, while one meat exchange would consist of 4 g TAG. As with carbohydrate counting, an insulin TAG ratio can be developed. The advantage of the TAG system compared with carbohydrate counting is that it includes the available glucose from animal protein. When used alone, neither TAG nor carbohydrate counting takes into consideration the fat or vegetable protein calories of the diet. However, in most situations this will not have a large effect on blood glucose levels (185). No matter what system is used for the nutrition component of intensive diabetes therapy, it should be obvious that a nutritionist familiar with the other components of the program is essential. The nutritionist needs to work with the patient in addition to the other team members to help develop the appropriate insulin algorithms and insulin: carbohydrate, or insulin TAG ratios, when appropriate. Finally, since the various nutritional interventions have different features and complexities, the program used will have to depend both on the nutritionists experience and the patients needs and capabilities.
RETINOPATHY & HYPOGLYCEMIA RISK Human UL Does NOT Work, Beef UL Does Table 1 Table 2 Table 3 Table 4 Table 5 Table 6 Table 7 Table 8 Figure 1 Figure 2 Figure 3 Figure 4 RISKS OF INTENSIVE DIABETES THERAPY Worsening of Retinopathy Several studies, including the DCCT (2), have re ported worsening of retinopathy with rapid introduction of improved glycemic control. It initially appeared that this deterioration in retinopathy was transient and benign, and over time, eyes in patients treated with intensive diabetes therapy fared better or at least no worse than those in conventionally treated patients (192195). However, it appears that this worsening of retinopathy is not necessarily self-limited and may progress to proliferative disease or even blindness (196,197). It was recently reported that the patients with the highest initial levels of glycosylated hemoglobin had the highest risk of blindness after 1 year of intensive diabetes therapy (197). It also appears that the greater the decrement of glycosylated hemoglobin level (during a 10-month period), the greater the risk of progression of retinopathy (198). The mechanism of this worsening in retinopathy is not known for sure, but may be a consequence of retinal ischemia and/or retinal glucopenia (199,200). Retinal blood flow is increased with chronic hyperglycemia (201), and it is proposed that the retina may reduce glucose uptake from the bloodstream (202) as occurs with the brain (203). Abrupt lowering of glycemia may then deprive the retina of nutrients if retinal blood flow then diminishes. This may account for the areas of retinal infarction (soft exudates) that are observed when glycemic control is quickly improved. Because of all the beneficial effects of improved glycemic control on diabetes complications, it would be inappropriate to recommend that patients not lower average blood glucose levels if preexisting retinopathy is present, especially in the absence of significant proteinuria or neuropathy. However, until further studies examining this issue are conducted, it would seem prudent to recommend a slow reduction of glycosylated hemoglobin levels to <2%/year (197, 198) with regular examinations by an ophthalmologist. Whether prophylactic laser coagulation should be considered in this patient population (197) merits further investigation. Hypoglycemia In the DCCT, the risk of severe hypoglycemia was more than threefold higher in the intensive therapy group, and this risk was correlated with mean glycosylated hemoglobin level (2). Some studies in adults (3,204) and children (205,206) have shown similar results, although others have not (207,208). Nevertheless, it appears that attempting euglycemia or near normal glycemia interferes with the recognition of hypoglycemia and the generation of protective responses against it (209). Symptoms of hypoglycemia and catecholamine responses are initiated at lower blood glucose levels when glycosylated hemoglobin levels are decreased to near the normal range (210.211). It appears that the primary etiology of this phenomenon is hypoglycemia per se. In patients with IDDM, experimental hypo glycemia will result in an elevated glycemic threshold (lower blood glucose level required to elicit the response) for counterregulatory hormones and hypoglycemic symptoms after recent hypoglycemia (212,213). Similar results have been reported in non-diabetic subjects (214). Consistent with these findings is the fact that in patients with insulinomas, impaired counterregulatory responses are normalized after surgical removal of the tumor (215). The problem of nocturnal hypoglycemia deserves special mention. Patients with IDDM and nondiabetic subjects have a similar decrease in insulin requirements in the early part of the night and then an increase in insulin requirements at dawn (20). The true prevalence of nocturnal blood glucose levels decreasing below 3 mmol/l in IDDM is unknown (20). Since autonomic symptoms may not be sufficient to awaken the patient (216), a mild episode of hypoglycemia could progress to severe hypoglycemia. In the DCCT, 43% of all severe hypoglycemia occurred between midnight and 0800 (217). Furthermore, asymptomatic nocturnal hypoglycemia may also con tribute to hypoglycemia unawareness (218). Attempts to decrease the risk of nocturnal hypoglycemia should include a regular bedtime snack, regular SMBG at bedtime and at least weekly in the middle of the night, and an insulin program that limit nocturnal hyperinsulinemia. These are the exact recommendations used for the intensive therapy group in the DCCT (32) where, as noted, nocturnal hypoglycemia was quite common (217). When the data are reported, it will be interesting to compare thc different rates of hypoglycemia in general and nocturnal hypoglycemia in particular with the different insulin regimens used in the DCCT. Theoretically, the use of CSII should provide the safest and most rational overnight insulin management (20). Secondary analysis from the DCCT (2) and data from Fanelli et al. (219) suggest that even mild increases in average blood glucose levels can decrease the frequency of severe hypoglycemia. The report of Fanelli et al. suggests hypoglycemia unawareness associated with meticulous glycemic control may be reversible, although a consideration is that this study included only eight patients with a relatively short duration of IDDM (s7 years). Confirmation of this finding and research for further strategies to prevent this problem are needed. From a practical viewpoint, a compromise in glycemic goals should be considered if severe hypoglycemia becomes problematic.
CONCLUSIONS Insulin therapy has evolved dramatically since the first description by Joslin et al. (1). On the other hand, many of the original concepts of how to manage IDDM have remained unchanged. Certainly, our tools for managing glycemia are more sophisticated, and perhaps most importantly, the rationale for meticulous glycemic control is indisputable. Unfortunately, our better understanding of how to implement intensive diabetes therapy carries with it the realization that it is quite difficult to accomplish. Our current open-loop strategies of insulin delivery are far from perfect, and it is obvious that meticulous attention to detail is critical for a successful outcome. For this reason, intensive diabetes therapy cannot be recommended for all patients with IDDM. Patients need to have the financial resources and be willing to perform frequent SMBG. They also need sufficient diabetes education and technical ability to follow a program of intensive diabetes therapy. The DCCT should be considered the model in which frequent interaction and education between patients and diabetes educators resulted in an improved outcome. Thus, skilled health care professionals must be avail able for implementation of intensive diabetes therapy. Finally, patients must be psychologically stable. Patients with unstable psychiatric disease or severe mental retardation would not be good candidates for an intensive diabetes therapy program. There are several challenges for the future. We must first focus on how to translate intensive diabetes therapy to the general population of patients with IDDM. Perhaps this can best be accomplished by "training the trainers," whether physicians, nurses, or nutritionists (220), and by better Using of the team approach (46). We also need further research on how we can best decentralize high-quality diabetes care (221). Early reports show that this can be successful (222). Improvements for the prevention of severe hypoglycemia must also continue to receive attention since this is clearly the greatest risk for the majority of patients. Finally, further Improvements in our technology to attain normoglycemia must continue (161,167). Improved insulin preparations (223), non-invasive glucose sensors (224), implantable insulin pumps (161), and perhaps, a closed-looped system could be available for all patients with IDDM within the next few years. For now, appropriate patients with IDDM should have the opportunity to implement intensive diabetes therapy. Note added in proof: Since this manuscript was written, the beef Ultralente insulin preparation has been discontinued. ACKNOWLEDGMENTS The author warmly acknowledges the staff of the University of Washington Diabetes Care Center and the hundreds of patients and their families who have educated him in the management of IDDM. Special thanks are extended to Drs. Jay S. Skyler. Philip E. Cryer, Julio V. Santiago, David E. Goldstein, Patrick J. Boyle, and Jerry P. Palmer. This manuscript would not have been possible without the support, collaboration, and enthusiasm of Ruth Farkas-Hirsch. References FOR THE REMAINING 213 REFERENCES, PLEASE CHECK THE ORIGINAL ARTICLE: Dr. Irl Hirsch, February 1995 Diabetes Reviews p. 288-307 RETINOPATHY & HYPOGLYCEMIA RISK Human UL Does NOT Work, Beef UL Does Table 1 Table 2 Table 3 Table 4 Table 5 Table 6 Table 7 Table 8 Figure 1 Figure 2 Figure 3 Figure 4 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||