Transfer to Human
Insulin is a Potential Risk
Factor for Severe Hypoglycaemia
Matthias
Egger*, George Davey Smith**,
Arthur Teuscher*
* Diabetes
Section, Department of Medicine,
University of Berne, Inselspital,
3010 Berne, Switzerland
** Department of Epidemiology
and Population Sciences, London
School of Hygiene and Tropical
Medicine, London, UK
published
in: Hypoglycaemia and Human
Insulin. Editors: Konrad
Federlin - Harry Keen - Hellmut
Mehnert. Georg Thieme Verlag
Stuttgart - New York 1991
Abstract
Prompted
by the clinical observation that
some patients with insulin
treated diabetes mellitus had
more difficulties to recognize
hypoglycaemia after transfer to
human insulin, a case-control
study of hospital admissions for
severe hypoglycaemia was
performed:
Risk
of severe hypoglycaemia in
insulin treated diabetic
patients transferred to
human insulin:
A case control study. British
Medical Journal
1991; 303:617-621
The period
from 1984 to 1987, when many
patients were unselectedly
transferred to human insulin
because porcine insulin became
no longer available, was
investigated. Compared to animal
insulin use, human insulin use
was associated with a
hypoglycaemia rate ratio of 2.4.
In order
to investigate whether the
symptom pattern associated with
human insulin hypoglycaemia
differed from that with porcine
insulin, a prospective
randomized double-blind
crossover trial was subsequently
performed:
Influence
of human insulin on symptoms
and awareness of
hypoglycaemia: A
randomised
double blind crossover
trial. British
Medical Journal
1991; 303:622-626
With human
insulin, patients were more
likely to report neuroglycopenic
symptoms such as lack of
concentration, restlessness,
confusion and visual
disturbance, but less likely to
report autonomic symptoms like
hunger, tremor and sweating.
Transfer to human insulin may
increase the risk of severe
hypoglycaemia. This may be due
to the symptom pattern
experienced in human insulin
hypoglycaemia which could impair
patients' ability to recognize
hypoglycaemia.
The
clinical observation that some
patients had more difficulties
to recognize hypoglycaemia after
transfer to human insulin (1,2),
and a number of episodes of
severe hypoglycaemia which
occurred after transfer to human
insulin (I) prompted us to
investigate whether transfer to
human insulin carried an
increased risk of severe
hypoglycaemia. The prospective
randomized clinical trial (RCT)
has been advocated to clarify
this issue (3). However, as
shown in Table 1, the
sample size requirements in a
RCT are considerable (4,5). For
example, in order to detect a 50
% increase in the risk of severe
hypoglycaeinia (relative risk
1.5) when assuming that 5 % of
patients experience one episode
per year, approximately 2,840
patients would have to be
followed up for one year.
Case-control studies require
smaller sample sizes (Table
1), however, they may be
subject to selection bias if
patients at high risk for
hypoglycaemia were more likely
to be transferred to human
insulin (6).
Table
1.Sample
size requirements in studies
investigating the risk of severe
hypoglycaemia during treatment
with human and animal insulin.
|
Study
type
|
Assumptions
|
Sample
size
|
|
Randomized
clinical trial
|
Follow-up
period 1 year,
incidence with
animal insulin
5/100 person
years, relative
risk to detect:
1.5
2.0
|
2.840
1.160
|
|
Case-control
|
60 % o
patients in the
population
treated with
animal insulin,
40 % with human
insulin, 2
controls per
case, relative
risk to detect:
1.5
2.0
|
1.380
408
|
All
calculations are based on a type
1 error of 5 % and a type 2
error of 20 %.
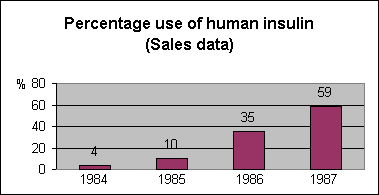
In
Switzerland this was not the
case because patients were
generally transferred to human
insulin purely because porcine
insulins were no longer
available. This resulted in a
rapid increase of human insulin
use during the years 1986 and
1987 (Figure 1). The
prescriptions gave the name of
the insulin (e.g. Actrapid® or
Monotard ® Novo), and when the
patients attended the pharmacy
to collect their insulin, they
would be given the human rather
than the porcine form. This
period when many patients were
unselectedly transferred to
human insulin can only be
investigated in a case-control
study.
Figure
1.
Percentage use of human insulin
as assessed from sales data,
1984 to 1987.
A hospital
based case-control study
of admissions for severe
hypoglycaemia to eight
Bernese public hospitals between
1984 and 1987 was therefore
performed. A computerized
registry was used for
identification of admissions for
hypoglycaemia (case admissions).
Control admissions were chosen
among insulin treated diabetic
patients admitted for a wide
range of other conditions. A
matched analysis, using
conditional logistic regression,
was performed (7). The odds
ratios obtained from this
analysis estimate the incidence
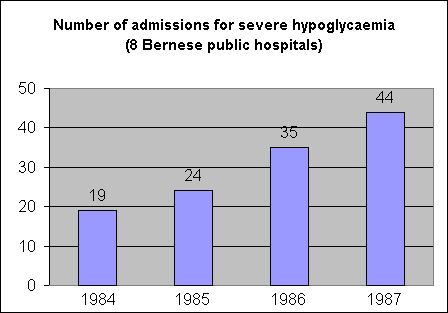
rate ratio (8,9). As shown in Figure
2, the annual number of
admissions for hypoglycaemia
increased from 1984 to 1987
(p<0.00l with Chi-square test
for trend in proportions). Case
and control admissions were
similar with respect to age,
sex, diabetes duration, onset of
diabetes, insulin dose, number
of daily injections, and body
mass index. Mean age was 51
years, mean diabetes duration
was 16 years. Therapy with human
insulin was more frequent among
case admissions than among
control admission. The relative
rate estimate for human insulin
as compared to animal insulin
was 2.4, indicating a 2.4 fold
higher rate for admissions with
severe hypoglycaemia in human
insulin treated patients. Other
risk factors included tight
glycaemic control, history of
prior hypoglycaemic coma and
early diabetes onset.
Figure
2. Number
of admissions for hypoglycaemia
(total number of admissions) to
eight public hospitals, Canton
of Berne, Switzerland, 1984 to
1987. p<0.001 with chi-sqare
test for trend.
In order
to investigate whether transfer
to human insulin alters
hypoglycaemia symptoms in a way
that may impair recognition and
appropriate response, a randomised
double-blind crossover study was
subsequently performed.
Forty-four patients with
insulin-dependent diabetes
mellitus (IDDM) (mean age 36
years, mean diabetes duration 16
years) were randomised to
initially receive human or
highly purified porcine insulin.
Twelve patients had experienced
recurrent hypoglycaemic coma
prior to the study. The trial
lasted for 12 weeks with
crossover to the other insulin
after six weeks. Hypoglycaemia
was defined as a blood glucose
value < 2.8 mmol/l. After
recovery from hypoglycaemia a
standardized symptom
questionnaire was completed.
There were no statistically
significant differences in blood
glucose profiles, haemoglobin
Alc levels, fructosamine levels
and insulin doses between the
two treatment periods. The
frequencies with which symptoms
appeared were investigated for
treatment with human and porcine
insulin. With human insulin
patients were more likely to
report neuroglycopenic symptoms
such as lack of concentration,
restlessness, confusion and
visual disturbance, but less
likely to report autonomic
symptoms like hunger, tremor and
sweating. Table 2 ranks
the symptoms according to the
frequency of occurrence during
treatment with human and porcine
insulin.
In order
to investigate which symptoms
may be helpful for recognition
of hypoglycaemia, and which
symptoms may impair awareness,
the twelve patients with a
history of recurrent
hypoglycaemic coma were compared
to the remaining 32 patients.
Patients with recurrent
hypoglycaemic coma were older,
had a longer diabetes duration
and lower levels of haemoglobin
Alc and fructosamine. For this
comparison, the overall symptom
frequencies over both treatment
periods were calculated. There
were marked differences in the
occurrence of hypoglycaemia
symptoms between the two groups (Table
3). Lack of concentration,
confusion and visual disturbance
were more frequently noted by
patients with a history of
recurrent hypoglycaemic coma,
whereas sweating, tremor and
hunger were less frequently
reported.
Table
2.Hypoglycaemic
symptoms ranked according to
frequency of occurence during
treatment with human and porcine
|
Human
insulin (n = 44)
|
Porcine
insulin (n = 44)
|
|
1. Lack
of concentration
2. Tremor
3. Restlessness
4. Sweating
5. Hunger
6. Confusion
7. Visual
disturbance
|
1.
Tremor
2. Sweating
3. Restlessness
4. Lack of
concentration
5. Hunger
6. Confusion
7. Visual
disturbance
|
Table 3.
Hypoglycamic symptoms
ranked according to frequency of
occurence in patients with and
without history of recurrent
hypoglycaemic coma
Recurrent
hypoglycaemic coma:
|
Yes (n
= 12)
|
No (no
= 32)
|
|
1. Lack
of concentration
2. Restlessness
3. Confusion
4. Visual
disturbance
5. Sweating
6. Tremor
7. Hunger
|
1.
Tremor
2. Restlessness
3. Sweating
4. Lack of
concentration
5. Hunger
6. Confusion
7. Visual
disturbance
|
Conclusion
We have
offered evidence that transfer
to human insulin may increase
the risk of severe
hypoglycaemia. This may be due
to the symptom pattern
experienced in human insulin
hypoglycaemia which is similar
to the one experienced by
patients with recurrent
hypoglycaemic coma. Patients
should be transferred to human
insulin under a physician's
guidance only and not in the
pharmacy because their porcine
preparation had been withdrawn.
They should be informed about
the possibility of a change in
hypoglycaemia symptoms. Since
human insulin in general has no
advantages over highly purified
porcine insulin (l0, ll), the
costs and benefits of universal
transfer of patients to human
insulin should seriously be
considered.
References
1. Berger,
W., Althaus, B.: Reduced
awareness of hypoglycaemia after
changing from porcine to human
insulin in IDDM. Diabetes Care
1987; 10: 260-61.
2.
Teuscher, A., Berger, W.:
Hypoglycaemia unawareness in
diabetics transferred to human
insulin. Lancet 1987; ii:
382-85.
3. Cryer,
P.: Human insulin and
hypoglycemia unawareness.
Diabetes Care 1990; 13: 536-37.
4. Egger,
M., Teuscher, A., Berger, W.:
Hypoglycaemia unawareness: human
vs. animal insulin. Diabetologia
1988; 31: 453-54.
5.
Schlesselman, J.: Sample size
requirements in cohort and
case-control studies of disease.
Am J Epidemiol 1974; 99: 381-84.
6. Sackett,
D.: Bias in analytic research. J
Chron Dis 1979; 32: 51-68.
7. Hosmer,
D., Lemeshow, S.: Applied
logistic regression. New York:
John Wiley & Sons, 19ß9.
8.
Greenland, S., Thomas, D.: On
the need for the rare disease
assumption in case-control
studies. Am J Epidemiol 1982;
116: 547-53.
9.
Rodrigues, L., Kirkwood, B.:
Case-control designs in the
study of common diseases:
updates on the demise of the
rare disease assumption and the
choice of sampling scheme for
controls. Int J Epidemiol 1990;
19: 205-13.
10.
Sonnenberg, G., Berger, M.:
Human insulin: much ado about
one amino acid? Diabetologia
1983; 25: 457-59.
11.
Pickup, J.: Human insulin. Br
Med J 1986; 292: 155-7.
|


