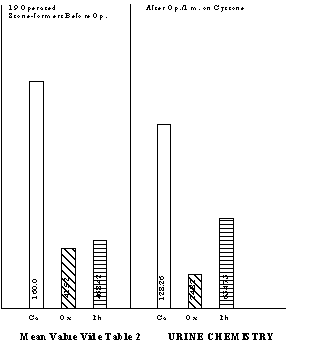|
(Indian Drugs, (1983): 7, 264) Blood and Urine Chemistry of Stone Formers in
Local Population and P.P. Singh, A.P. Pendse, Alka
Goyal, Rita Ghosh, A.K. Srivastava Depts. of Biochemistry & Surgery, R.N.T. Medical College,Udaipur (Rajasthan), India. INTRODUCTION Urinary stones constitute a major health problem in Rajasthan. As reported earlier the surgical incidence of cases is more than 50 per 10,000 hospital admissions in the Udaipur region. This places it in the high incidence category as per ICMR classification. Furthermore, the incidence has been progressively rising in the last years. Ironically we have yet to understand the aetiology of the disease and unravel the reason for its high prevalence in the region. Broadly, three theories have been advanced to explain stone formation. First, that organic colloidal material is necessary to initiate the process of stone formation and that the organic matrix plays a key role in the growth of stones11. Second, that it is a process of spontaneous precipitation of inorganic crystalloids at a urinary pH when these salts cannot be related any further17,20. Third theory lays stress on the significance of "Crystal Poisons" 4. Their decrease results in stone formation. The latter two theories are most favoured. Today, the majority view held by urologists and biochemists is that urinary crystalloid imbalance is the major cause of stone formation. Pyrah17 has rightly said that we should deploy our efforts, whether long-term or short-term, to remove intra-renal or extra-renal causes contributory to stone formation and not merely accept the patients as finally cured, when a stone has been voided spontaneously or removed surgically. It is this growing rationale which has placed increasingly greater reliance on biochemical investigations in calculus disease. There is also awareness that metabolic aberrations can provide the final clue to the disease in most of the patients with stones, if not all. These biochemical abnormalities are quite often reflected in the blood and urine. As such the first step to explore the aetiology of stone disease should be to thoroughly investigate the blood and urine chemistry for stone-forming and stone-inhibiting substances. STONE-FORMERS AND STONE-INHIBITORS The emerging picture shows that urine is usually supersaturated with crystalloids. Some crystalloids like calcium, oxalic acid and uric acid have a tendency to get precipitated in the urine conduit to form stones. On the other hand, substances like phosphates, magnesium, sodium, potassium and many others help to hold the stone-forming crystalloids in solution. These substances are broadly classified as "Crystal Poisons", and they inhibit crystal formation in the urine, thereby minimising the chances of stone formation or stone growth. Among such stone-inhibitors present in the urine are phosphates (the most important) magnesium, sodium, potassium etc. When the stone growth is inhibited, chances of voiding the stone are also more. A subtle balance between stone forming and stone preventing substances is maintained in normal urines. Any adverse tilt in the balance can result in the formation of a stone. Raised excretions of calcium, oxalic and uric acid (Hypercalciuria, hyperoxaluria and hyperuricosuria) increase should be brought down. On the other hand, the excretion of crystal poisons (stone-inhibitors) should be increased. Thus, any conservative remedy for urolithiasis must keep this twin-purpose in view. A drug which can decrease the concentration of stone-forming substances (crystalloids) and/or increase the concentration of stone-inhibitor substances (colloids/crystal poisons) in the urine should prove of value for prophylaxis or to avoid recurrence in operated cases and to inhibit further growth of performed stones. Its likely value, in disintegrating the calculus in situ and expulsion per urethra should also be assessed. In recent years, Cystone of The Himalaya Drug Co. has gained increasing usage in the conservative treatment of urolithiasis and has been claimed to offer multiple benefits2,3,7,15. COMPOSITION Each Cystone tablet contains :
Hajrul yahood bhasma is prepared with Ocimum basilicum, Tribulus terrestris, Mimosa pudica, Dolichos biflorus, Pavonia odorata, Equisetum arvense, Tectona grandis seed. The present paper deals with the blood and urine chemistry of healthy volunteers as well as stone-former cases from the local populace and effect of Cystone on the latter. MATERIAL AND METHODS Seventy-four persons were included in this study. Of these, 19 were healthy volunteers (16 males and 3 females) and 55 uroliths (46 males and 9 females). The uroliths had reported to the Surgical Outdoor of the General Hospital, R.N.T. College, Udaipur, with complaints indicative of urolithiasis. They were admitted to the surgical ward and the presence of stone was radiologically confirmed. After admission in the ward, all the patients were advised to take the routine diet. Their detailed case history including clinical assessment, socio-economic status and dietary habits, were recorded by verbal questionnaire method. After two days, 24-hour urine sample (8 a.m. to next day 8 a.m.) was collected in 2.5 litre bottles containing 10 ml conc. HCL. ‘Twenty-four hours’ urine volume was recorded and the sample was analysed for creatinine10, calcium12, oxalic acid8, uric acid28, total phosphrous13, magnesium13, sodium and potassium (Flame Photometry). The pH of the fasting urine was checked by narrow range BDH pH papers for three consecutive days. The blood sample of all the subjects was collected in plain vials (5 ml). Serum was analysed for creatinine10, calcium13, magnesium13, inorganic phosphorous13, uric acid28, and alkaline phosphatase14. Following laboratory investigation of all the patients, they were divided into 2 groups. The first group of 25 males and 4 females had stones smaller than 1 cm in size and were not in imminent need of surgery. They were as such put on conservative (non-surgical) management in the expectation that the stone may descend and be expelled. For investigative purposes, the cases were grouped as follows :
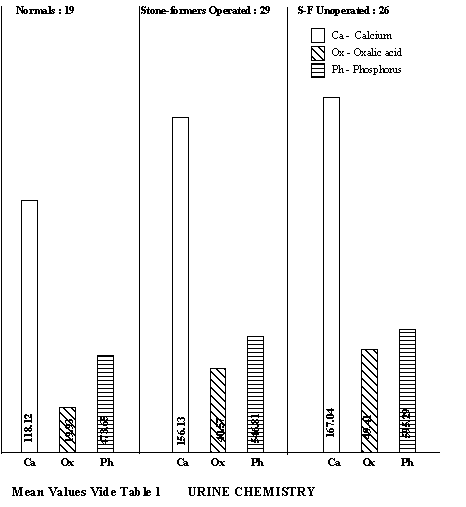
After laboratory investigations, the two latter groups were placed on Cystone 2 tablets three times a day. They were called for a check-up after completion of the first and the second month on Cystone. OBSERVATIONS As the population in this region is largely vegetarian, maize and wheat was the staple food of most persons. Only one subject was a regular non-vegetarian. A prominent feature was the consumption of oxalate-rich leafy vegetables (amaranth, spinach, pigweed etc.) during season. However, the patients had discontinued the consumption of green leafy vegetables including the above as soon as they suspected stone disease. Furthermore, all of them were taking liberal quantities of water at the time of their reporting in the hospital. Infact, the cases have become self-motivated enough to take several precautions, as urolithiasis has been widely prevalent in this region for a long time. The initial observations on the urine chemistry in respect of normal persons, operated and unoperated stone-formers are summarised in Table 1. The urinary output in stone-formers (both operated and unoperated) was higher than normal. This increase was due to larger intake of water as these patients are encouraged to take plenty of water as soon as symptoms of stone disease are suspected.
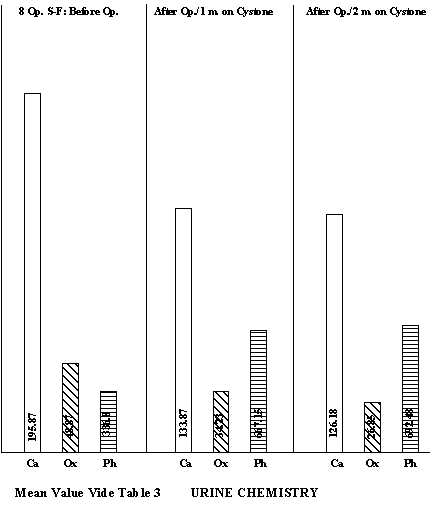
In general, the calcium excretions were higher in all stone-formers, though statistically significant only in the unoperated cases. An important feature was hyperoxaluria in the stone-formers (p<0.001). The uric acid excretion in general was on the lower side of normal. So also, the excretions of stone-inhibiting substances such as total phosphorous and magnesium were on the lower side. RESULTS Nineteen of the 29 operated stone-formers turned up for the first check-up and eight of them for the second. The results obtained in them with Cystone are summarised in Tables 2 & 3 respectively. It was observed that Cystone treatment had decreased the calcium and oxalic acid excretion and at the same time increased phosphorous excretion. Similar response to Cystone was evident among unoperated stone-formers where calcium excretion dropped from 187.10 ± 79.68 mg% to 132.10 ± 52.11 mg% and oxalic acid from 53.45 ± 23.16 mg% to 42.97 ± 20.39 mg% (Table 4).
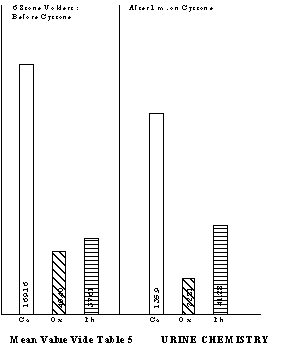
Seven of the unoperated stone-formers voided the stone spontaneously, following Cystone therapy. Six had ureteric calculi and one had vesical calculi. Two had been operated in this hospital (earlier on) and now passed the stone within 1 to 2 weeks of Cystone treatment. Urinary profile in respect of six patients is given in Table 5. This shows that after Cystone treatment, the mean values of calcium and oxalic acid decreased considerably and that of phosphorous increased, although the differences were not statistically significant.
A classification of normal subjects and stone-formers on the basis of 24-hour urinary excretions of calcium, oxalic acid, uric acid and total phosphorous appears in Tables 6-9. Sixteen stone-formers had calcium excretions > 200 mg/day while 21 had oxalate excretion > 50 mg/day. Hyperuricosuria does not appear to be a problem in this region. The most characteristic feature was hypophosphaturia.
Prior to Cystone, a general tendency towards increased calcium excretion and strikingly high incidence of hyperoxaluria in stone-formers was very clearly seen, as also lower excretion of total phosphrous. Following Cystone treatment, a gradual shift from the higher range of calcium and oxalic acid excretions to lower range to and from the lower range of phosphorous excretion to the higher range in all groups of stone formers can be precisely seen. These results clearly bring out the beneficial role of Cystone in maintaining an improved crystalloid balance between crystal-formers and crystal poisons. The post-treatment analysis of the blood chemistry showed that Cystone corrected hypercalcaemia and hyperalkaline phosphatasemia in a majority of patients (Tables 10-16). Thus, Cystone fulfills the necessary therapeutic criteria expected of a conservative remedy for urolithiasis.
DISCUSSION Increasingly greater reliance is being placed on biochemical investigations in calculus disease as metabolic aberrations can provide the vital clues to the presence of the stone disease in most cases. As biochemical abnormalities are quite often to be found in the blood and urine, in the last two decades persistent efforts are being made to investigate these two fluids to track down the stone-forming substances. It is increasingly clear that a urine supersaturated with stone-forming crystalloids like calcium, oxalic and uric acid is prone to form stones, unless excretion levels of stone-inhibitors like phosphates, magnesium, sodium and potassium hold the stone-formers in check to prevent precipitation of stones in the urine. Any crystalloid imbalance between stone formers and stone poisons results in stone disease. Similarly, blood chemistry can also provide valuable information in this respect. Calcium is notorious for its renal tubular damage and stone-forming propensity. A tendency towards increased calcium excretion was very much apparent in stone-formers in our series. Hypercalciuria (>200 mg/day) was observed in 24.1% of the operated and 34.61% of the unoperated stone cases. Chally et al.,1 observed hypercalciuria in 57.1% stone formers and 20% normal subjects. High percentage of hypercalciuria in stone-formers has also been reported from Manipur and some parts of North India. Jackson and Dancaster9 feel than an otherwise normal hypercalciuric population could be potential stone-former due to this risk of calcium in urine. These observations and of many others make it abundantly clear that hypercalciuria should be controlled at the earliest instance. Hyperoxaluria, either of endogenous or exogenous origin, is equally or even more, dangerours17,21,22,24,26. It has two-fold disadvantages. Firstly, oxalate supersaturation can trigger off calcium oxalate precipitation and secondly, it can induce hypercalciuria which can aggravate the situation. Thus hyperoxaluria appears to be still more serious hazard in the local population. The percentage of uroliths who excreted 40 mg oxalic acid/day was 58.26 for operated and 73.07 for unoperated. About one third of uroliths excreted 59 mg oxalic acid/day. The role of urinary phosphates, magnesium and sodium in the prevention of calculus formation has greatly been stressed in the recent years. Sodium ions are believed to replace calcium ions in the crystal lattice of salt thereby decreasing the formation of crystals. Potassium ions should play a similar role and therefore its excretion should be taken into account along with sodium. The data in the present series show normal excretions of sodium and potassium in both normal subjects and uroliths; magnesium excretion is slightly on the lower side of normal and phosphorous excretion is definitely low in all groups. At present we are unable to offer any exact explanation for this but presumably it could be due to cereal-rich vegetarian diet which may contain as high as 70-80 per cent phytin phosphorous. The latter is unavailable for absorption. It can be made out from these results that hyperoxaluria and hypophosphaturia are important determinants in the causation of this disease in the local population and efforts should be made to eliminate these adverse conditions. The urinary oxalates appeared to be of endogenous origin as, at the time of hospital admissions, patients were already on lower oxalate diet. Subsequently we planned to evaluate the influence of Cystone treatment on uroliths. The results of one month Cystone treatment in 19 operated patients are given in (Table 2). There was no significant difference in urinary volume and pH before and after the treatment. However, there was a mild increase in urinary volume and an effort for readjustment of pH within the normal range. Calcium excretion was reduced form 160.0 ± 69.0 mg/day to 128.26 ± 49.06 mg/day but was not statistically significant. There was a significant fall in urinary oxalates (p<0.02) and significant rise in phosphorous (p<0.05). The effect of Cystone therapy on these parameters can still be better understood from a marked shift in the number of patients from higher range of calcium and oxalic acid to the lower range and in case of phosphorous, vice versa. Eight patients turned up for their second check up after two months and the redeeming feature of Cystone treatment was a significant fall in calcium (p<0.05) excretion, along with the oxalic acid (p<0.05). Increase in phosphorous excretion became still more significant. Among eleven unoperated Cystone-treated cases, no statistically significant difference was observed before and after treatment but it can be seen that Cystone does try to convert an evil urine to good urine. Six patients had both hyperoxaluria (>50 mg/day) and hypercalciuria (>200 mg/day) but after one month treatment they disappeared in four patients (Table 9). Seven patients voided the stone spontaneously during the process of treatment. At present it is too early to grant Cystone the status of a drug which would pulverise the stone in situ, but certainly the benefit of the doubt goes in its favour. Frankly, this drug should be given a trial in a larger series. Out of 26 unoperated cases 15 did not turn up for check up. Whether this was because they got rid of the trouble during the treatment could not be verified. Several workers have stressed that urine pH can provide important information on the genesis and type of stones present in the urinary tract5,17. Ready precipitation of uric acid occurs at pH 5.0 or below, of oxalate between 5.0 to 6.5, and of phosphates 7.5. In 79.31% of operated and 73.07% of unoperated uroliths the urinary pH was in the range of 5.0 to 6.5. This gives an inkling that calcium oxalate crystals should have provided the nucleus for stone formation. This is further supported by the fact that hypercalciuria and hyperoxaluria was present in large number of patients. This being so, Cystone should prove as a potent drug to control recurrence of the stone disease. Blood chemistry of stone formers showed elevated levels of creatinine in 4 urolithis which was corrected after surgery. Hypercalcaemia, a very undesirable feature, was present in 14 uroliths (Table 15). All hypercalcaemic patients were tested three times for serum calcium, alkaline phosphatase and inorganic phosphorous levels. No patient was found to have high calcium and alkaline phosphatase levels and low inorganic phosphorous. Neither clinical findings nor these tests were suggestive of any frank case of parathyroid. Results of Cystone treatment on blood chemistry speak well in its favour though again a larger series should be tested to give a final verdict (Table 16). Hypercalcaemia disappeared in 4 operated uroliths after one month Cystone treatment and in the fifth one after two months’ treatment. Among 11 unoperated uroliths it disappeared in all the 3 hypercalcaemics after one month’s treatment. The drug also brought back alkaline phosphatase levels within normal limits in 50 per cent cases. Other parameters did not show any toxic effect on blood chemistry in respect of the parameters studied. SUMMARY
REFERENCES
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||