|
(Antiseptic (1979): 7, 393) Liv.52 IN THE TREATMENT OF CIRRHOSIS OF LIVER — CERTAIN OBSERVATIONS K.K. Singh, M.B.B.S., M.D. (M.U.), D.T.M. & H. (M.U.),Formerly Research Scholar, Department of Medicine, Y.K. Singh, M.B.B.S. (B.U.), M.D. (M.U.),K.M. Dubey, M.D. (B.U.)Associate Professor, department of Biochemistry, V. Sharma, M.D. (Pat.),Assistant Professor, Department of Medicine and Prof. B.N. Mishra, D.T.D. (Delhi), M.R.C.P. (U.K.),Professor & Head of the Depat. of Medicine, Darbhanga Medical College, Laheriasarai, INTRODUCTION Cirrhosis of the liver is a generic term applied to chronic diffuse liver disease of multiple aetiology characterised by (i) destruction of parenchymal cells (ii) distortion of normal lobular architecture with nodular regeneration of the parenchymal cells and (iii) overgrowth of fibrous tissue. The Liver controls numerous metabolic processes; acts as a storage organ; synthesises plasma proteins, fibrinogen, prothrombin, etc.; secretes bile and detoxicates various substances. So the disease of the liver are bound to have adverse effects on the entire system. Cirrhosis of liver occurs all over the world but is common in developing countries where malnutrition prevails. Cirrhosis of the liver peculiar to the tropics is either directly due to a relative and absolute deficiency of protein or indirectly by predisposing the liver to noxious substances such as toxins, parasites and viruses. Hepatotoxins of pyrolizidine group and aflatoxins cause cirrhosis. Aetiological factors causing cirrhosis of the liver are various e.g., hepatic infection, diet, alcohol, autoimmunity, obstruction to the bile ducts, chronic congestive cardiac failure, hepatic infiltrations. There are three basic pathological types of cirrhosis: i. Diffuse hepatic fibrosis (portal cirrhosis). ii. Post-necrotic cirrhosis. iii. Biliary cirrhosis. The resulting complications of hepatic failure and coma or haemorrhagic diathesis with its ultimate fatality occur quite frequently in our country. The disease is among those which as remained unconquered even in the Western countries. A satisfactory specific treatment is not yet available. Liv.52, a herbal-mineral preparation, is said to have diuretic, anabolic, aperient, choleretic, stomachic, haematinic actions with protective and regenerative properties (Sule et al, 1956; Murkibhavi and Sheth, 1957; Northover et al, 1960; Joglekar et al, 1963; Captain and Syed, 1966; Damle and Deshpande, 1966; Kale et al, 1966; Joglekar and Leevy, 1970; Patel et al, 1972). Marked clinical improvement as well as improvement in liver function tests brought about by Liv.52 was observed in cases of severe hepatic damage (Sule and Sathe, 1957), in diffuse hepatic fibrosis and portal hypertension (Mathur, 1957). In a few cases of infantile cirrhosis decrease in cellular infiltration and necrotic changes were noted by Vyas in 1960. Its effect on liver cell regeneration and protection against hepatotoxicity has been described by many workers (Joglekar, G.V. and Leevy, C.M.; Kulkarni, S.D. et al, 1971; Patel, Jal R. and Sadre, N.L., Prasad, G.C. 1974). Liv.52 has been shown in various animal studies as well as clinical trials to bring about regeneration of healthy liver parenchyma. The exact mode of action of Liv.52 is not fully known, but it is evident from the findings of various workers that it has definite protective and regenerative actions on the liver parenchyma in more ways than one. The ultimate clinical picture of the disease (cirrhosis of liver) is dependent on the degree of underlying hepatocellular damage, (Rickels et al, 1948; Popper et al, 1950, Jhingam et al, 1965; Dasgupta and Mukerjee, 1970). Each Liv.52 tablet contains:
This trial of Liv.52 in cases of cirrhosis of liver has been undertaken to determine the therapeutic efficacy of the drug in the light of highly encouraging claims of various authors. MATERIAL AND METHOD The present study on 80 patients of cirrhosis of liver was carried out at the Darbhanga Medical College Hospital, Laheriasarai. The age of the patients who belonged to both the sexes, ranged from 15 to 65 years. The diagnosis of cirrhosis of liver was made on the basis of clinical features, routine investigation of blood, urine, stool and other specialised investigations like plasma proteins (total plasma proteins and albumin and globulin separately), serum bilirubin, serum alkaline phosphates, thymol turbidity, S.G.P.T., S.G.O.T., cytology and chemistry of ascitic fluid and liver biopsy. Patients having haematemesis and maelaena were excluded from the study. Patients were divided into two groups of 40 each. One group was put on Liv.52. The schedule dosage was six tablets daily. The other group served as control and was treated with Vit. B-complex, corti-costeroids and diuretics. Each patient was examined thoroughly at intervals of 2 months, 4 months and 6 months - including symptomatology, physical signs and specialised investigations to determine the degree of improvement in both the groups as well as to compare the merits of the two lines of treatment. Cases in each group were initially examined histopathologically and then after 2 months, 4 months and 6 months of treatment. For clinical evaluation each group was divided into advanced, established and early cases on the basis of the histopathological picture (Table I). The efficacy of the treatment was judged by the amelioration of subjective symptoms and objective signs, the degree of improvement in various liver function tests, as well as the degree of histopathological improvement.
OBSERVATIONS AND RESULTS In the present study of 80 cases of cirrhosis of liver of varying severity - early, established and advanced cases (on the basis of histopathological picture) 62 were males and 18 were females ranging from 15 to 65 years of age, the majority (34%) falling in the age group of 36 to 45 years (Table II).
Of various symptoms, loss of appetite was present in 56 cases and flatulence in 51 cases. Various other features like diarrhoea, vague abdominal pain, epigastric discomfort, jaundice, nausea, vomiting, pruritus, dark coloured urine, fever, anaemia, weakness etc. were also observed (Table III). Of the physical signs, venous prominence was seen in 76 cases. Ascites, hepatomegaly, splenomegaly, oedema, testicular atrophy, gynaecomastia, spider angiomata were also found in varying percentages (Table IV). The progress of the patient was evaluated clinically, by different biochemical tests for assessing liver function and by liver biopsy. Symptomatic improvement in the Liv.52 treated series occurred much earlier and most of the symptoms (about 75%) disappeared after 6 months of therapy and the rest of symptoms showed marked improvement; in the control group most of the symptoms (58%) showed a rising trend. The other symptoms either remained static or declined.
RESULTS - LIVER FUNCTION TESTS Serum bilirubin Average initial values of serum bilirubin in the control and Liv.52 groups were 1.72 mg% and 1.33 mg% respectively. These values after 2 months of treatment in the two groups were 1.78 mg% and 1.28 mg% and after 4 months of treatment 1.85 mg% and 1.18 mg% and after 6 months of treatment 1.93 mg% and 0.87 mg% respectively. Sub-icteric level of bilirubin was seen before treatment in the control group in only 80% and in the Liv.52 group all cases showed lower subiceteric levels (Table V).
Serum alkaline phosphatase: The initial level ranged from 5-38 K.A. Unites with an average of 16.20 K.A. Units. in the control group. In the Liv.52 group it was 6-36 K.A. Units with an average of 15.8 K.A. Units. The average values after 2 months, 4 months and 6 months of treatment in the control group showed 16.40, 19.45 and 20.90 K.A. Units whereas in the Liv.52 group the values showed 14.7, 14.07 and 11.92 (Table V). Thymol turbidity The initial range of thymol turbidity in the control and Liv.52 groups was 3-14 units and 1-8 units with an average of 5.07 units and 5.32 units respectively. The average values after 2 months, 4 months and 6 months of treatment in control group were 6.7 units, 7.52 units and 8.75 units as against 4.45 units, 4.2 units and 3.47 units respectively in the Liv.52 group (Table V). S.G.P.T. The average initial values in the control and Liv.52 groups were 51.4 and 54.3 units respectively. In the control group after treatment for 2 months, 4 months and 6 months average values rose to 54.5, 57.97 and 61.2 units respectively, but in the Liv.52 group average values showed a declining trend (Table V). S.G.O.T. The initial average values of S.G.O.T. in the control group was 53.7 units as against 51.67 units in the Liv.52 group. In the control group this rose to 57.7 units, 62.5 units and 67.2 units after treatment for 2 months, 4 months and 6 months respectively whereas in the Liv.52 group the values declined to 48.7 units, 42.95 units and 29.17 units after treatment for 2 months, 4 months and 6 months respectively (Table V). Total plasma protein The initial level ranged form 4.7 to 6.9 g% with an average of 5.395 g% in the control group and from 5.5 g% to 7.0 g% with an average of 5.815 g% in the Liv.52 group. The average values after 2 months, 4 months and 6 months of treatment in the control group were 5.417 g%, 5.447 g% and 5.47 g% as against 5.865 g%, 5.972 g% and 6.145 g% respectively in the Liv.52 group (Table V). Plasma albumin The initial plasma albumin level ranged from 1.5 - 3.9 g % with an average of 2.50 g% in the control group as against 1.9-4.4 g% with an average value of 2.52 g% in the Liv.52. The average values in the control group after 2, 4 and 6 months of treatment were 2.36 g%, 2.24 g% and 2.08 g% whereas in the Liv.52 group they were 2.75 g%, 3.09 g% and 3.56 g% respectively. Plasma globulin The initial average value in the control and the Liv.52 groups were 2.895 g% and 3.3 g% respectively. In the control group after treatment for 2 months, 4 months and 6 months, values rose to 3.05 g%, 3.21 g% and 3.382 g%. In the Liv.52 group after treatment for 2 months, 4 months and 6 months values declined to 3.107 g %, 2.877 g% and 2.585 g% (Table V). Albumin: Globulin ratio The A : G ratio which was 1/1.16 and 1/1.32 in the control and Liv.52 groups respectively at the beginning of the study, reversed in the Liv.52 group after treatment to 1.37/1, while in the control group the value of albumin diminished and that of globulin increased and so the ratio became 1/1.62 (Table VI).
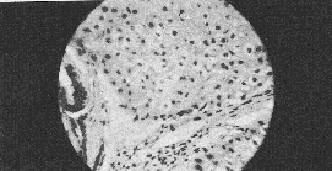
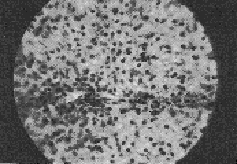
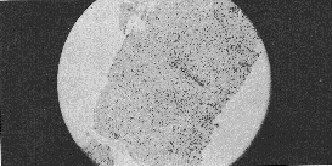
Histopathology This initial histopathological picture in both the control and Liv.52 groups showed three types of histological picture (I) slides showing cloudy swelling, fatty infiltration, cell degeneration and at few places, fibroblastic activities (labelled as early cirrhotic changes), (ii) slides showing cell degeneration, fatty infiltration definite fibrotic bands at few places with intact architecture (labelled as established cirrhosis cases) and (iii) with massive hepatic fibrosis, formation of pseudolobule and complete loss of architecture (labelled as advanced cirrhosis cases). After treatment in the control series hepatocellular damage actually advanced and in a few cases there was insignificant change in the histological picture. The Liv.52 groups after treatment showed definite improvement in the histological picture with aggregation of cells at places, denoting regeneration, was seen in 2 cases after treatment with Liv.52. Very few cases remained unchanged. The best result was observed after treatment for 6 months. DISCUSSION The results in the present series indicate that treatment with Liv.52 has a definite advantage over conventional therapy. In Liv.52 treated cases symptoms like anorexia, weakness, nausea, vomiting, disappeared probably due to the anabolic effect of Liv.52 (Kale et al, 1966; Damle and Deshpande, 1966). Amelioration of the other most distressing features like flatulent dyspepsia, oedema, ascites etc. was also due to the anabolic and diuretic action of Liv.52. Vague abdominal pain and epigastric discomfort were also relieved satisfactorily in the majority of Liv.52 treated cases but not in the control cases. These symptoms are difficult to explain but are probably due to associated gastritis and ascites.
Diarrhoea is due to fat indigestion as there is lack of bile in the duodenum. It was controlled after treatment, indicating that the secretion and excretion of bile increased after Liv.52 therapy. As regards the liver function tests, overall improvement of these was clearly noted after 6 months of treatment with Liv.52 but patients in the control group showed progressive deterioration. Serum bilirubin came to sub-icteric level in all the patients while serum alkaline phosphatase, thymol turbidity, S.G.P.T., S.G.O.T., remained above normal limit in 10, 7, 8 and 2 patients respectively in the Liv.52 group while values of these tests in the control group remained elevated in most of the cases or remained static in a few cases. The flocculation test like thymol turbidity is dependent on the abnormal pattern of plasma protein distribution as a result of disturbed hepatic synthesis of serum albumin. A diminution in the thymol turbidity value therefore shows a corrective trend of this aberration by Liv.52 - presumably by improving the functioning state of the hepatocytes as also by promoting regeneration of the necrosed cells, thereby improving the protein synthesis. Reversion of albumin/globulin ratio from 1/1.32 to 1.37/1 is in favour of this. Another reflection of the cell-protective and regenerative function of Liv.52 is the progressive diminution of the serum enzymes like S.G.P.T. and S.G.O.T. Such regeneration and repair of liver cells by Liv.52 has also been observed by Vimala Ramalingam et al, 1971, and Prasad G.C., 1974. Post-treatment liver biopsy reports show marked improvement after 6 months of treatment with Liv.52 while control patients showed deterioration. Mukerjee and Dasgupta (1971) have suggested a period of 9 months for optimum effect of the therapy. In this series, since it was felt that our patients would not always turn up for the follow up, it was decided to study the effect for a shorter period of therapy of 6 months - though it cannot be denied that a more prolonged therapy of 9 months or more would have been more beneficial to the patient than 6 months. Therapy with Liv.52 also shows a marked and significant improvement in the overall condition. The use of an indigenous drug Liv.52 on confirmed cases of cirrhosis of liver has been evaluated both clinically and by various liver function tests, biopsy, etc. Liv.52 was found to have definite value in the treatment of cirrhosis of liver, especially early cases. A minimum period of 6 months of treatment was found to be necessary for significant improvement. Control treatment showed deterioration in all the early, established and advanced group of cases. Out of 9 early cases of cirrhosis of liver in the test group, 8 cases showed marked clinical and biochemical improvement. This was also confirmed by the biopsy after six months. In the control group, out of 6 early cases, one showed mild improvement, one was static and four deteriorated. In the established cases in the test group, out of seventeen cases there was clinical and biochemical improvement in ten cases and the histopathological improvement was found in seven cases. In the control group, out of 12 established cases, two were static and ten deteriorated. Similar findings were observed on histopathological examination. In the test group, there were 14 advanced cases. Out of these six showed satisfactory improvement in the clinical and biochemical findings. In three cases the liver biopsy revealed definite structural improvement. In the control group, there were 22 advanced cases, of which one was static and 21 deteriorated. SUMMARY Eighty histologically proven cases of cirrhosis of the liver were studies by dividing into two groups of 40 patients. One group received Liv.52 2 t.i.d. while the other received standard therapies such as diuretics, corticosteroids, vitamins etc. The Liv.52 treated cases showed markedly superior clinical improvements which were borne out by biochemical liver function tests and histopathological assessment after 6 months of therapy. The control cases, on the other hand, deteriorated clinically, biochemically and histopathologically. ACKNOWLEDGEMENT We are thankful to the Superintendent, Darbhanga Medical College Hospital for allowing us to conduct this work and for permitting us to publish it. We are also thankful to Dr. A.B. Khan, M.D., Associate Professor of Pathology, for his valuable suggestions and guidance. REFERENCES
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||