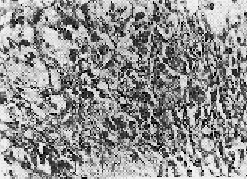|
(Asian Medical Journal (1982): 9, 647.) ROLE OF LIV.52 IN INDIAN CHILDHOOD CIRRHOSIS, WITH SPECIAL REFERENCE TO ITS EFFECT ON ALPHA-I-ANTITRYPSIN LEVELSNaval Kishore Agarwal , M.D., Ph.D., M.A.G.S.,Lecturer in Physiology, R. Prasad, M.D., M.R.C.P., D.C.H.,Prof. of Clinical Paediatrics, Manju Sharma, M.Sc.,Research Scholar and B.B. Sharma, M.D., M.A. (Toronto),Ex.Prof. & Head of the Dept. of Physiology, and Principal and Chief Superintendent, S.N. Medical College and Hospital, Agra (U.P.), India. ABSTRACT A study was conducted on 23 cases of Indian Childhood Cirrhosis (I.C.C.) to assess the effectiveness of treatment with Liv.52. Serum Alpha-I-Antitrypsin (Alpha-I-AT) was estimated with M-Partigen immunodiffusion plates, using a Radial immunodiffusion method. The pre-treatment levels of serum Alpha-I-AT were significantly low in these cases. 6 cases had heterozygous levels (60-180 mg%) while 2 case had homozygous deficiency(<60 mg%). These cases were administered Liv.52 alone, in dose of 20 drops 3 times a day, for a year. No other medication was given during the period. At the end of one year on Liv.52, 56.5% cases were adequately controlled and showed clinical and biochemical improvement. Serum Alpha-I-AT levels were significantly higher (P < .01) in both non-deficient and deficient cases of cirrhosis following one year’s therapy with Liv.52. L:iv.52 acts as a hepatotonic and an anabolic drug. It relieves cholestasis and improves organic anion transport. This herbal preparation can therefore be given safely in the routine treatment of cirrhosis.
COMPOSITION Each ml of Liv.52 drops contains:
INTRODUCTION Indian Childhood Cirrhosis is a fatal condition which involves mainly children of younger age groups. The condition seems to occur especially in first child and more particularly in the first male child, following the birth of several female children1,2,7. Its aetiology is unclear and therefore, it is difficult to treat and to cure. Many reports have associated Alpha-I-AT deficiency with the aetiopatho-genesis of the disease.3,6,8,11 Liv.52 (The Himalaya Drug Co.) is a hepatotonic which has been widely used to protect the liver from hepatotoxic agents and in many chronic liver diseases5,10. Singh and Agarwal (1978)12 have demonstrated the significant role of Liv.52 in the treatment of I.C.C. The aims of the present study were to assess the efficacy of Liv.52 therapy in I.C.C. cases and to determine the correlation, if any, with Alpha-I-AT in such cases. MATERIAL AND METHOD A total of 23 patients were included in the present study. Of these, 20 were males and 3 females, their median age being 2.13 ± 0.69. The cases were diagnosed and graded according to the I.C.M.R. classification (1955). 4 Before initiating treatment, the cases were examined in detail and liver function tests were carried out. Alpha-I-AT was estimated by the radial immuno-diffusion method of Mancini et al (1965)9 using the M-partigen immuno-diffusion plates of Behrringwerke, Germany. Alpha-I-AT deficiency was assessed in each case according to the criteria outlined in our earlier studies6-8. (Figs. 1, 2 and 3).
Liv.52 was administered in daily doses of 20 drops orally, 3 times a day upto one year. During this period , no other drugs (antibiotics, corticosteroids or diuretics) were given to these patients. The efficacy of the therapy was assessed by clinical, biochemical and Alpha-I-AT estimations at 3 months’ intervals throughout the treatment period. The student ‘t’ test was used for statistical analysis.
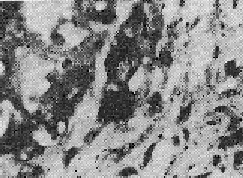
OBSERVATIONS Of the 23 patients, 20 (87%) were males and 3 (13%) were females. 15 (65.2%) were below 2 years of age, 7 (30.4%) between 2 and 5 years and one was over 6 years (4.4%). A familial history of the disease could be traced in 30.4% of the cases on the paternal or maternal side. More than 50% were first born male children. According to the I.C.M.R. (1955) grading, 87% cases belonged to Grade 3 while 13% were Grade 2 cases. The mean level of Alpha-I-AT in the 23 cases was 161.5 ± 76.8 mg/100 ml. 8 cases revealed Alpha-I-AT deficiency. 6 cases were heterozygous whereas 2 were homozygous. The mean Alpha-I-AT level among the non-deficient was 193.4±32.6 mg%, while in the heterozygous and homozygous groups, the levels were 120.5±39.6 mg% and 41.0 mg% respectively. The diagnosis in each case was confirmed by the histopathological study of biopsy material from the liver. Liver biopsies were performed with a Wilms Silverman needle. The material was fixed, stained by standard staining methods and examined microscopically, (Figs. 4 and 5) to evaluate the cirrhotic stage. Such biopsies revealed cirrhotic degenerative changes (Grade II). Intracytoplasmic globule infiltration could be seen in the specimens studied.
RESULTS Fifteen cases (65.2%) completed the full course of the trial. Of these, 13 cases (56.5%) showed an overall improvement within one year’s duration while 2 cases (8.6%) showed no noteworthy progress. Eight cases (34.9%) expired during the period. CLINICAL ASSESSMENT With Liv.52 therapy, jaundice was relieved in 55% cases. Symptoms of vomiting, diarrhoea and abdominal distension were absent after one year. In 82% of cases, appetite had improved. The liver regressed in 39% and the spleen was not palpable in 60% (Table I).
Biochemical Assessment The results of liver function tests carried out before and during the trial (at 3 months intervals) as shown in Table II. The administration of Liv.52 brought about a significant fall in serum bilirubin, serum alkaline phosphatase (SAP), total leucocyte count, ESR and gamma globulin whereas there was a significant rise in the values of haemoglobin, total serum proteins and serum albumin. The Albumin: Globulin ratios also reverted towards normal values. The results indicated that the functional incapacitation of the liver caused by cirrhosis was very much less in the Liv.52 treated cases (Table II).
Alpha-I-AT Estimation Prior to the treatment, the cases of Indian Childhood Cirrhosis had shown lower levels of Alpha-I-AT in their sera. Of the 8 cases of Alpha-I-AT deficiency (< 180 mg%) recorded in the present study, 6 were heterozygous (range: 60-180 mg%) and 2 cases were homozygous (< 60 mg). Following the treatment with Liv.52, serial estimations of Alpha-I-AT revealed an overall increase in deficient as well as in non-deficient cases. The Alpha-I-AT levels were increased significantly (P<0.01) in the heterozygous deficients. In the homozygous cases, however, Alpha-I-AT estimations could not be repeated since both the patients expired within a week (Table III).
DISCUSSION Alpha-I-AT is a low molecular weight glycoprotein which is synthesised in the liver. Its deficiency is apt to be familial, probably with autosomal recessive mode of heritability11. This protein inhibits proteolytic enzymes such as trypsin, chymotrysin, elastase and collagenase. In deficiency states, the proteolytic enzymes become active and damage the liver. This association of Alpha-I-AT deficiency with liver diseases has been confirmed by several earlier workers6-8,11. It would seem, therefore, that a drug which can stimulate the liver to synthesise more Alpha-I-AT or release the protein from hepatic cells, may prove useful in the management of liver disease. Liv.52 is claimed to have a protective and regenerative effect on the hepatic parenchyma- as a choleretic which has a salutary effect on liver glycogen and serum proteins, along with diuretic and anabolic actions as well (Patney et al)10. In view of this, Li.52 was investigated in cases of I.C.C. with special reference to its effect on Alpha-I-AT levels. The results of our one-year study on Liv.52 in the treatment of 23 cases of Indian Childhood Cirrhosis indicated that Liv.52 improves the functional capacity of the liver and significantly relieves the presenting clinical symptoms. The liver function tests also showed a remarkable improvement. Serum bilirubin and serum alkaline phosphatase (SAP) were reduced by 50% indicating that Liv.52 relieves cholestasis and improves organic anion transport. The anabolic activity of Liv.52 resulted in an increase in the total serum proteins and serum albumin concentration. With the administration of Liv.52 the Alpha-I-AT levels increased significantly (P < .01), both in the deficients and nondeficients. Liv.52 probably stimulates the liver to synthesise more of this glycoprotein and then releases it into the circulation. The resultant increase in level of glycoprotein (Alpha-I-AT) protects the liver from the proteolytic enzymes which may damage the liver3,6,8,11. The most notable finding was that once the patients obtained relief, the salutary effect of Liv.52 remained stable for the remainder of the period of study. This clearly indicates that drug tolerance to Liv.52 does not develop and hence a schedule of increasing dosage is not required. No adverse reactions were noted in any of the patients during the one year period. To sum up, the clinical biochemical and immunochemical assessments made in this study show that Liv.52 is an effective and well-tolerated treatment without any attendant toxicity and has an important place in the management of Indian Childhood Cirrhosis. ACKNOWLEDGEMENT We are grateful to The Himalaya Drug Co., Bombay (India) for the assistance and supply of Liv.52. REFERENCES
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||