 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
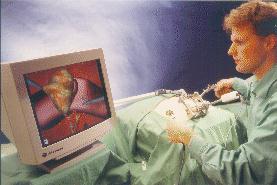
The word 'robot' evokes many different thoughts and images, perhaps conflicting ones. Some may think of a metal humanoid, others of an industrial arm, and yet more may think, unfortunately, of a lost job. In the field of medical robotics, the word robot is just as fuzzily defined, with many different applications. These range from simplistic laboratory robots, to highly complex surgical robots that can either aid a human surgeon or execute operations by themselves. The reasons behind the interest in the adoption of medical robots are multitudinous. There is a great analogy to be found with the automation involved in the manufacturing industry. That is not to say that the issues of medical robotics are the same, but that the advantages to be gained are similar. Robots provide industry with something that is, to them, more valuable than even the most dedicated and hard-working employee - namely speed, accuracy, repeatability, reliability, and cost-efficiency. A robotic aid, for example, one that holds a viewing instrument for a surgeon, will not become fatigued, for however long it is used. It will position the instrument accurately with no tremor, and it will be able to perform just as well on the 100th occasion as it did on the first. The applications of robots in medicine will be further expounded, and the field of robotic (and robotically assisted) surgery will be concentrated upon, along with such issues as safety and implementation. In the future, maybe sooner than we think robots may help doctors improve their ability to diagnose and treat disease. Whether a doctor is at a patient's bedside or is miles away, the doctor may have a better sense of "feel" inside the body. Operating rooms of the future may look a little different, because a robot might be doing some of the work.
Some robots can be as small as or smaller than human cells. This is the field of nanotechnology. The use of microscopic robots is emerging as the next technological revolution. It looks at building materials and devices with atomic precision. Imagine the broad implications for the future of medicine.
Robotic surgery is the process whereby a robot actually carries out a surgical procedure under the control of nothing other than its computer program. Although a surgeon almost certainly will be involved in the planning of the procedure to be performed and will also observe the implementation of that plan, the execution of the plan will not be accomplished by them - but by the robot. The advantages to be gained through automation are numerous. A robot's motions can be precisely controlled and constrained through its programming. This results in undeviating trajectories, high accuracies with predictable velocities and accelerations with no overshoot. As expected when dealing with automated processes, the benefits of repeatability and reliability are inherent. An improvement is also experienced in terms of time. Unlike a human surgeon, a robotic one will not hesitate before each step, contemplating the possible outcomes of the next move. Some might say that this is a disadvantage of robotic surgery, but these outcomes will have been considered and reconsidered by surgeons in the pre- operative phase and so do not require further deliberation. Others may have the opinion that time will be wasted on the planning of the surgical procedure and on the imaging requirements. These, however, are necessary steps of any proposed surgery and, anyhow, the time saved in both the quicker procedure and in the reduced recuperation time will outweigh any increase in the pre-operative stage. In order to look at the different issues involved in the robotic fulfilment of an operation, the separate sections of a typical robotic surgery (although robotic surgery is far from typical) are explained below.
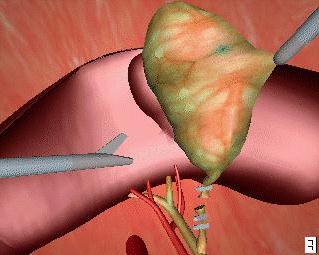
Surgical planning consists of three main parts. These are imaging the patient, creating a satisfactory three-dimensional (3D) model of the imaging data, and planning/rehearsing the operation. The imaging of the patient may be accomplished via various means. The main method is that of computer tomography (CT). CT is the process whereby a stack of cross-sectional views of the patient are taken using magnetic-resonance-imaging or x-ray methods. This kind of imaging is necessary for all types of operative procedure and, as such, does not differ from traditional surgical techniques. This two-dimensional (2D) data must then be converted into a 3D model of the patient (or, more usually, of the area of interest ). The reasons for this transformation are twofold. Firstly, the 2D data, by its very nature, is lacking in information. The patient is, obviously, a 3D object and, as such, occupies a spatial volume. 2D data is just that - two-dimensional; hence it cannot easily provide information pertaining to such issues as volume (of, for instance, a tumour) or, position (with respect to distances perpendicular to the cross-sectional data). Secondly, it is more accurate and intuitive for a surgeon, when planning a procedure, to view the data in the form that it actually exists. The actual transformation into a 3D model is readily accomplishable through volume graphics methods (see Volume Graphics: The road to interactive medical imaging?). These methods produce computer-graphics-based models that possess such features as the ability to rotate the model, view its interior, zoom in, and so on. That is, all the capabilities of current computer-aided-design (CAD) systems. As may be expected, however, the processing requirements of these modelling systems are rather large, as are the costs of the hardware necessary. It should be noted, however, that the speed of said hardware is increasing all the time and the price will decrease too, as the technology involved becomes more commonplace. This means that the process will be more cost-efficient and increasingly routine in the future. The third phase of the planning is the actual development of the plan itself. This involves determining the movements and forces of the robot in a process called 'path planning' - literally planning the paths that the robot will follow. It is here that the 3D patient model comes into play, as it is where all the measurements and paths are taken from. This emphasises the importance of the accuracy of the model, as any errors will be interpreted as absolute fact by the surgeons (and hence the robot) in their determination of the plan. Here, surgeons are reliant upon the engineering behind the system that is being used - thus the need for reliable systems (discussed in the section on safety).
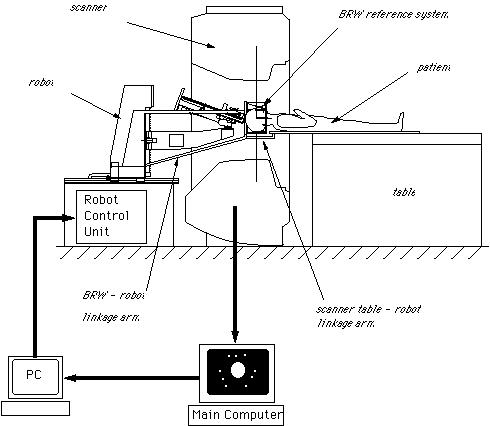
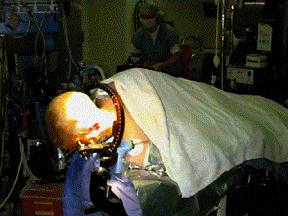
There are two important stages in the registration procedure - fixation of the patient and the robot, and intra-surgical registration itself. Fixation is an essential ingredient of a successful robotic operation. Robots act upon pre-programmed paths (as mentioned above), these programs are much more complex (and more difficult to prove for safety aspects) if they must take into account the fact that the patient's position may be different to the inputted data and, in fact, continually changing. For this reason it is imperative that the robot can act in, at least, a semi-ordered environment. Fixation of the patient, that is fixing the patient in position (i.e. on the operating table), is achieved through strapping and clamping of the areas pertinent to the surgery. This is common in traditional surgery, too. For example, the leg of a patient undergoing a knee operation is clamped in position to avoid unintentional movement; similarly, the head is fixed in position during neurosurgery through the application of a head-fixation device known as a 'stereotactic unit'. Fixation of the robot is achieved through analogous methods. Patient held in fixed position for neurosurgery The intra-surgical registration itself is the process of establishing a common reference frame between the pre-surgical data (3D model and associated surgical plan) and the corresponding patient anatomy. Once a common reference is established, pre-surgical data can be safely used to guide robotic movements.There are two primary techniques of achieving this common frame of reference. The first, and most usual, method is to attach fiducials (physically implanted markers) to the underlying patient structures pre- operatively (and necessarily before imaging takes place). These fiducials are then sensed, and compared to the pre-operative data, to precisely align the two data sets. Unfortunately, this 'fiducial- based' registration typically requires an additional surgical operation in itself, in order to attach the markers. Furthermore, these fiducials are invasive and cause added trauma to the patient in sites physically far from the primary field of surgical focus. The alternative to fiducial-based registration is that of 'surface- based' registration. This technique uses surfaces that are intrinsic to the data itself. If surfaces can be extracted from both the pre- and intra-surgical data, then these data sets can be matched to perform registration. The benefit of this method is that it does not require the use of expensive and traumatically invasive markers. The success of surface-registration is highly dependent upon the realism and accuracy of the 3D models gained pre-operatively and upon the sensing accuracy of intra-surgical data acquisition. Geometric surface model validation is complicated since errors can be introduced at several stages of model creation: during imaging, 'segmentation' (seperation of different tissues within a medical image), and surface creation. Prior to the emergence of surface-based techniques for surgery, 3D modelling of medical data has been primarily used as a teaching aid in the study of anatomy (e.g. VOXEL-MAN). These models have very different accuracy requirements to those used for surface-based registration. For instance, the ease with which an object of interest (e.g. a particular organ) can be segmented from surrounding objects is of crucial importance in robotic surgery since any 'blurring' of edges could mean that erroneous incisions are made. Another demand, placed upon the geometric model of the patient, is the ability to cope with 'spatial density' variation within an object. Density variations, like those in real bones and tissue, complicate segmentation by reducing the effectiveness of simple thresholding schemes (methods that interpolate a density from the surrounding data and assign the density value according to certain, pre-determined, thresholds).
Once all of the preparation is complete, it is time to pass control to the robot for the actual implementation of the surgery. The robots used for automated surgery tend, at the present time, to be adapted industrial robots; for example, the PUMA robot arm. The reasons behind this are predominantly financial ones. Investing in the research and development of a robot for a specific task is highly expensive - costs running into millions of pounds, with no guarantee that a suitable product will be the outcome. Another hurdle is the lack of official safety guidelines and standards. Having brought up the fact that the surgeon is reliant upon the engineering behind the tools that they are using, it is clear that companies would not wish to risk possible legal proceedings should one of their products fail. (These issues will be further discussed in the section on safety). As it stands, industrial robots are adequate for the tasks involved, although it is hoped (and expected) that, in the future, surgery- specific robots will be developed and will become an industry of their own. Once the robotic procedure is initiated, sensors collect real-time data from the operating site and pass this to a display, via which the surgeon observes the operation. The type of sensor that seems to best suit the surgical application is the use of infra-red transmitters on the robot's 'joints', that are detected by cameras in the operating theatre (currently under commercial development by General Electric). From these, the exact position of the robot's appendages can be gauged and relayed back to the surgeon's display. It is an issue as to what control a surgeon should have when overseeing an automated surgery - as they may do more damage than good if they intervene. The most common arrangement is to have a 'stop' switch and perhaps a 'redo' control. Sensing the patient, intra-surgically, is fairly simple when using fiducials (the fiducials are specially made from materials that are easily detectable under the system being used). This is not the case, however, when using the preferred surface techniques. Here a variety of systems are under consideration, including an ultrasound sensor or an x-ray sensor - both mounted on a 'C-arm' so that they can move around the whole area of the patient.
Evaluation is an integral part of any surgical operation. In a robotic one, it is even more so since the surgeon did not have an opportunity to check everything before proceeding with the subsequent steps. If it is found that something is not completely satisfactory, then the surgeon has the option to either repeat the whole operation or to carry out a manual procedure to complete the work. It has been shown that, in the operations conducted robotically, robots can produce superior results to human surgeons. For example, the milling of a socket for an artificial hip replacement has been performed with greater accuracy (in terms of 'fit' and 'contact' of the prosthesis) than a human surgeon can perform. In particular, one of the main criteria for success in this type of operation is the percentage of the prosthesis that is in contact with the bone of the socket. Robotic surgeons have managed up to 83% as compared to around 30% for humans. This, on the surface, dramatic improvement is tempered by the fact that a different approach (not using robotics) has also yielded 83% contact. This approach uses humans to mill the socket and then custom-produces the artificial hip to fit the shape of the socket. Thus, the robotic solution must be able to show some other improvement (e.g. cost- or time-wise) if it is to replace the human surgeon altogether. This argument holds in every surgical (and medical) robotics application. The robotic method must posess great benefits, not only in terms of performance, but also in terms of cost-effectiveness, ease of use and other such factors. The robot must also be psychologically acceptable to both surgeons and their patients, as there have been cases where a surgeon has refused to use a robot because of its appearance - 'looks too clumsy/menacing' etc. If all of these requirements are met, then the advantages of robotics that manufacturers have experienced for years can be transferred to the operating theatre, increasing the welfare of patients, and surgeons, the world over.
In comparison to robots used in the industrial sector, medical robots present designers with much more complicated safety problems. Some of the most important factors which lead to such complexity are described below:
Possible reasons that can lead to unsafe operation of a medical unit include flawed design, malfunction of hardware and software components, misinterpretation and incorrect or inadequate specification. As in many other applications, improving some of these parameters results in a degraded performance in other areas, while an overall increased level of safety is accompanied by an increase in cost, complexity, or both. The idea of total safety is a fallacy. Instead, different safety strategies offer different advantages (and, or course, disadvantages). The overall probability of error must be always kept at very low levels. Perhaps even more important than the probability of a fault is the ability to detect that a fault has indeed occurred and prevent hazards resulting from it, that is, allow the robot to "fail safely". This usually involves shutting the robot down and removing it from the patient, and having the operation manually completed by a surgeon. As the task which the robot undertakes becomes more and more complicated, there is an increasing need for more complex hardware and software components (faster response, better accuracy, more degrees of freedom). This increases the probability of error exponentially. Software is notoriously difficult to reason about, while hardware reliability never ceases to be of prime importance. A final consideration concerning safety is, perhaps surprisingly, size. Both patients and doctors feel uncomfortable working next to medical robots which tower above the surgeon at over 7 feet and weigh in at several tens of kilograms. There is some logic behind that, however. A larger robot can usually exert more force than a smaller one, resulting in an increased amount of damage in case of a fault.
Although there is a large amount of time and money invested researching this field, medical robot units are still not massively produced, resulting in prohibitively high costs for proprietary technology. A major problem is presented by the lack of an industry-wide accepted safety standard, partly because the definition of safety depends largely on the application, and partly because a rigid, too strict a specification would impede further pursuit towards that direction. In addition, there are no clear boundaries to the extent of the surgeon's and the manufacturer's responsibility in case of a fault, preventing medical robot manufacturers from having a clear picture of the situation. A final point that further adds to the problem is that robot manufacturers are reluctant to make all design specifications available to medical safety committees. It can be generally said that the safer a robot is, the fitter it is to work next to humans. Since complete safety is unattainable, however, the question arises: When is a medical robot safe enough? This greatly depends on the application. If it is to perform an ordinary operation, the robot's abilities must match if not surpass those of a surgeon. If it is to perform a life-saving operation that would be otherwise impossible, it is plausible that the safety requirements can be relaxed, since the patient would die anyway if the operation is not performed. This solves, nevertheless, only part of the problem. Life-saving operations are invariably complicated ones, resulting in the need for a robot with sophisticated capabilities, which, as mentioned earlier, results in a greater probability of error. The complications of this question go a lot deeper, the bottom line being that the surgeon is a human being, and, as such, is prone to errors as well. Examples of surgeons forgetting gloves or pairs of scissors in the patient or making unnecessarily deep incisions are not as rare as one would expect. So, a possible answer could be that the robot is safe enough when it is safer than the surgeon, or even that it is safe enough when the robot's failure is preferable to that of a surgeon.
Medical robotics, and particularly autonomous surgical robotics, are still in an embryonic stage. Concentrating on surgical robots, the reasons for their not gaining immediate enthusiasm and acceptance in the medical community are twofold. Issues of safety have been highlighted, in particular, as hurdles to research and development taking place. Safety issues have traditionally not been addressed and there is an urgent need for a concensus on what is 'safe practice' concerning both human-guided and autonomous robots. The first matter (that of safety) is a definite problem. Industrial robots operate in a confined 'cell of activity' that is seperate from their human counterparts - this obviously cannot be the case with surgical robots. This will require immediate action if it is not to further hinder the development of a field that can provide great benefits to society. Considering the fact that increased complexity, both in the program of an autonomous robot and in the design of a guided (or autonomous) robot, increases the problem of defining safety standards, it is the opinion of the authors' that the way forward in surgical robotics should be one that uses human-guided robots and/or powered robots that are extremely task specific. A robot that has all the skills of a human surgeon would be extremely complex; it is perhaps better to limit the abilities that the robot has and, in doing so, limit the possible damage it could do if it were to malfunction. The second matter is one of education and social conditioning; it should also be eased through the solving of the safety predicament. A good point to observe, for worried surgeons, is the fact that, at best, robots can (at the moment) provide a crude substitute to an expert surgeon. The human hand has twenty degrees of freedom, while the most advanced robots can only provide eight or nine. In addition, in robotic surgery, the ability to image and model anatomical structures has outperformed the ability to perform physical, robotic intervention. There have only been three research groups that have devised specific powered systems that have an autonomous cutting function (ISS - hip surgery, EPFL - neurosurgery, Imperial College - prostrate surgery). In the vast majority of robotic surgeries, the surgeon has control of tool-holders or positioning robots to improve their accuracy and perfomance. To conclude, there are several steps that must be taken in order to further the use and development of robots in surgey (and in medicine in general). These are:
The economic and social advantages to be gained from the mass-use of robotics in medicine (and particularly surgery), as already expounded, are enormous. If all of the above steps are taken, then the full potential of robotics can be exploited in the medical sector, as it has been in industrial applications, for the improved welfare of society everywhere.
|
| Back to Top |

 |