TABLE OF CONTENTS FOR DIF'S "Diabetics World"
Annual Adverse Drug
Experience Report:
1995
http://www.fda.gov/medwatch/safety/ar95.pdf
Deanne E Knapp, Janet I Robinson, and Annie L Britt
Surveillance
and Data Processing Branch
Division of Pharmacovigilance and Epidemiology
Office of Epidemiology and Biostatistics
Center for Drug Evaluation and Research
Food and Drug Administration
ACKNOWLEDGEMENT
The authors wish to thank Ms. Traci R. Tate for preparing the manuscript, graphs, and tables.
Annual Postmarket Adverse Drug Experience Report: 1995
Lilly Humulin 8th worst for Adverse Events
TABLE OF CONTENTS
ANNUAL ADVERSE DRUG EXPERIENCE REPORT: 1995.... . 1
ACKNOWLEDGEMENT...................................................... . 1
TABLE OF CONTENTS........................................................ 1
INTRODUCTION.................................................................. .. 2
TYPES OF ............................................................. .... 3
GEOGRAPHIC LOCATION OF INITIAL REPORTER............. ..... 5
SEX AND AGE OF PATIENTS................................................. 6
SERIOUS OUTCOMES........................................................... 7
LATENCY BETWEEN SUSPECT DRUG AND ADE ONSET....... 8
ROUTES OF SUSPECTDRUGS.............................................. 11
ABATEMENT OF ADVERSE EVENT....................................... 11
REOCCURRENCE OF ADVERSE EVENT.................. . ........... 12
BODY SYSTEMS................................................................... 12
ADVERSE EVENTS............................................................... 13
INTRODUCTION
This report presents a descriptive
overview of the 130,950 evaluable1,
postmarket adverse
drug experience (ADE) cases
received by the US Food and Drug Administration
(FDA) during
calendar year 19952. A
case consists of the original report of an ADE
on a patient plus
any followup2
information.
FDA has long conducted a program to
monitor ADEs for marketed drugs. As part of this
program, a computerized ADE database was begun
in 1969 and has accumulated about 1.2
million
cases. The primary purpose for maintaining the
database is to serve as an early warning
or
signaling system for ADEs not detected during
premarket testing. The ADE system depends
upon
detection of an adverse clinical event by a
health professional or consumer, attribution of
the
clinical event to prior administration of a
particular drug ("suspect" drug), and
reporting of the
ADE to the manufacturer of the
suspected drug or directly to FDA. Data from
these ADE cases
are coded and entered into the
computerized ADE database. Copies of the ADE
cases
are stored on microfilm or an imaging
system. Up to five drugs per case may be entered
into the
computerized ADE database; the five can
be a combination of "suspect" and
"concomitant"
drugs. Up to four
adverse events per case and their associated
body systems can be coded into
the database,
using FDA's "Coding Symbols for Thesaurus
of Adverse Reaction Terms"
(COSTART).
1. Excludes “React Uneval” cases.
2.
The 1995 postmarket ADE computerized data
file used for this report was created June
1996.
Reporting
of postmarket ADEs by health professionals and
consumers is voluntary. They may
send their
reports directly to FDA ("Direct"
reports), to the drug manufacturer
("Manufacturer"
reports), or both.
Drug manufacturers are required by law and
regulation to submit to FDA any
postmarket ADE
reports received by any means from health
professionals or consumers.
It is important to
remember certain caveats when using data from
FDA's postmarket ADE
database:
1.
For any given ADE case, there is no
certainty that the suspected drug
caused the
ADE. This is because physicians and consumers
are encouraged to report
all suspected ADEs, not
just those that are already known to be caused
by the drug. The
adverse event may have been
related to an underlying disease for which the
drug was
given, to other concomitant drugs, or
may have occurred by chance at the same time the
suspect drug was administered.
2.
Accumulated ADE cases may not be used to
calculate incidences or estimates
of drug risk.
Numbers from these data should be carefully
interpreted as reporting
rates and not
occurrence or incidence rates.
Over the next pages, various kinds
of data and information are presented on the
postmarket
ADE cases computerized into the FDA
ADE database during calendar year 1995. Due to
rounding, the percentages in tables and graphs
may not total to 100%. Figures 1 and 2 present
copies of the postmarket ADE forms used by
manufacturers and health professionals or
consumers, respectively [and the Figure:
3500A MedWatch Mandatory Form and Figure 3500:
MedWatch Voluntary Form are both excluded from
the FDA Report
located at http://www.fda.gov/medwatch/safety/ar95.pdf].
Top of Page
TYPES OF REPORTS
There are three types of reports in the FDA computerized postmarket ADE database:
1.
Manufacturer-reported cases concerning
ADEs not in present official FDA
labeling with
serious outcomes (i.e., death, life-threatening,
hospitalization, permanent
disability,
congenital anomaly, cancer, or overdose). These
cases are known in regulatory
language as
"15-day Alert Reports" because the
manufacturer has 15 working days to
submit this
type of report to FDA.
2.
All other manufacturer-reported cases.
These cases are known in regulatory
language as
"Periodic Reports" because the
manufacturer is required to submit them to FDA
on a cyclical basis.
3.
Cases sent directly to FDA by health
professionals or consumers ("Direct
Reports").
As shown in Figure 3, reports
submitted to FDA via manufacturers accounted for
88%
(115,663) of the 130,950 postmarket ADE
cases. Only 12% (15,287) were submitted directly
to
FDA. 15-day reports were 16% (20,434) of the
total.
REPORTING
BY HEALTH PROFESSIONALS
AND CONSUMERS
As shown in Figure 4, in 1995, for
a little over one-third of the 130,950
postmarked ADE
cases, consumers were the
original reporters. Figure 4 also shows that,
over a 3-year trend
(1993-1995), reports from
consumers have increased both in absolute
numbers and
proportionally whereas those from
health professionals have done the opposite.

Top of Page
GEOGRAPHIC
LOCATION OF INITIAL
REPORTER
As shown in Table 1, the initial
reporter for 83% (108,735) of the 130,950
postmarket
ADE cases was located within the US
or its territories. Nine percent (11,843) of the
cases were
missing location source. For the
108,735 US cases, the top-two ranked regions,
Middle and
South Atlantic, accounted for
two-fifths of the cases.
There were eight percent (10,372)
of the postmarket ADE cases where the initial
report
source was foreign. There were four
countries which each accounted for <=10% of
the foreign
cases: France (22%, 2,288), Japan
(14%, 1,490), Germany (13%, 1,310), and United
Kingdom
(12%, 1,289).
|
Table 1. Postmarket ADE Reports by Geographic Location of Initial Reporter: 1995 |
||
|
|
N |
% |
|
All Locations |
130,950 |
100 |
|
US CENSUS REGION: |
108,735 |
83 |
|
a Middle Atlantic |
21,507 |
20 |
|
South Atlantic |
21,264 |
20 |
|
East North Central |
16,748 |
15 |
|
Pacific |
14,498 |
13 |
|
West South Central |
9,003 |
8 |
|
West North Central |
7,246 |
7 |
|
New England |
6,664 |
6 |
|
Mountain |
6,204 |
6 |
|
East South Central |
5,475 |
5 |
|
Trust Territories |
126 |
<1 |
|
FOREIGN: |
10,372 |
8 |
|
b France |
2,288 |
22 |
|
Japan |
1,490 |
14 |
|
Germany |
1,310 |
13 |
|
United Kingdom |
1,289 |
12 |
|
UNKNOWN |
11,843 |
9 |
a US Census Regions are percentaged to 108,735
b Foreign Countries are percentaged to 10,372
Top of Page
SEX AND AGE OF PATIENTS
As shown in Table 2, the ratio of
female-to-male postmarket ADE cases was 1.6:1.
For
females, the 20-39 year age group accounted
for the greatest number of known sex-age cases,
whereas for males, it was the $60 year age
group.
|
Table 2. Postmarket ADE Reports by Sex and Age of Patient: 1995 |
||
|
|
N |
% |
|
All Sexes & Ages |
130,950 |
100 |
|
ALL FEMALES: |
73,341 |
56 |
|
<=19 yrs |
5,202 |
4 |
|
20-39 yrs |
18,311 |
14 |
|
40-59 yrs |
15,092 |
12 |
|
>= 60 yrs |
15,651 |
12 |
|
Unknown age |
19,085 |
14 |
|
ALL MALES: |
44,473 |
34 |
|
<= 19 yrs |
3,886 |
3 |
|
20-39 yrs |
6,485 |
5 |
|
40-59 yrs |
9,779 |
7 |
|
>=60 yrs |
14,298 |
11 |
|
Unknown age |
10,025 |
8 |
|
UNKNOWN SEX: |
13,136 |
10 |
|
<= 19 yrs |
284 |
<1 |
|
20-39 yrs |
120 |
<1 |
|
40-59 yrs |
174 |
<1 |
|
>=60 yrs |
241 |
<1 |
|
Unknown age |
12,31 |
9 |
Top of Page
SERIOUS OUTCOMES
As shown in Figure 5,
hospitalization was the most recorded serious
outcome; congenital
anomaly, the least.

Top of Page
LATENCY BETWEEN
SUSPECT DRUG
AND ADE ONSET
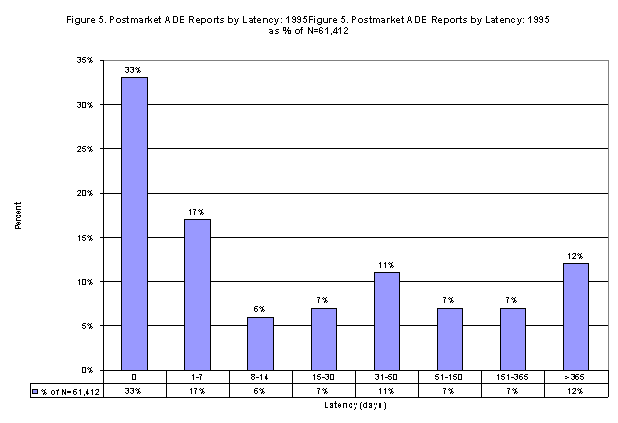
As
shown in Figure 6, of the 130,950 postmarket ADE
cases, 47% (61,412) had both a drug start date
and an
adverse experience onset date for the
first-listed suspect drug and the first-listed
adverse experience, respectively,
and where the
drug date was computerized as occurring before
the adverse experience date. About half of these
cases noticed the adverse event occurred within
one week of drug initiation.
CLASSES OF SUSPECT DRUGS
Annual
Postmarket Table 3 presents the top-10 ranked
drug classes associated with the 153,842 suspect
drugs
computerized from the 130,950 postmarket
ADE cases. The top-ranked drug class, central
nervous system agents,
accounted for
approximately one-quarter of the drug class
mentions3. Together with the 3 second
and third
ranked drug classes, hormones and
synthetic substitutes and cardiovascular drugs,
these top three ranked drug
classes comprised
about half of the total drug class mentions.
|
Table 3. Postmarket ADE Reports by Top-10 Ranked Classes of Suspect Drugs: 1995 |
||
|
|
N |
% |
|
All Suspect Drug Mentions |
153,842 |
100 |
|
Central Nervous System Agents |
42,254 |
28 |
|
Hormones & Synthetic Substitutes |
21,173 |
14 |
|
Cardiovascular Drugs |
15,711 |
10 |
|
Anti-Infective Agents |
14,117 |
9 |
|
Atineoplastic Agents |
10,191 |
7 |
|
Skin & Mucous Membrane Agents |
9,115 |
6 |
|
Autonomic Drugs |
6,658 |
4 |
|
Gastrointestinal Drugs |
5,589 |
4 |
|
Unclassified |
5,436 |
4 |
|
Blood Formation & Coagulation |
3,812 |
2 |
3.
The drug classification used was the
American Hospital Formulary Service
Pharmacologic - Therapeutic Classification
(American
Society of Health-System Pharmacists,
Bethesda, Maryland, 1996).
Top of Page
SUSPECT DRUGS BY ENTRY NAME
AND
NEW MOLECULAR ENTITY STATUS
Table
4 shows the top-10 ranked suspect drugs as
entered on the 130,950 postmarket ADE reporting
forms.
Three are nonprescription drugs, Aleve™,
Today™, and Humulin™
insulin. Three are used by
females (Norplant™,
Depo-Provera™, and Today™)
and one primarily by males (Rogaine™). New
Molecular Entities (NMEs) are defined
as new
drugs approved within the past three years. For
this 1995 report, NMEs are new drugs approved
during
1992-5. Of the 153,842 suspect drugs
computerized from the 130,950 postmarket ADE
cases, 15% (23,275) involved
NMEs.
|
Table 4. Postmarket ADE Reports by Top-10 Ranked Suspect Drugs: 1995 |
||
|
|
N |
% |
|
All Postmarket ADE Reports |
130,950 |
100 |
|
Aleve™ |
6,642 |
5 |
|
Norplant™ |
5,712 |
4 |
|
Prozac™ |
3,253 |
2 |
|
Depo-Provera™ |
2,647 |
2 |
|
Risperdal™ |
2,540 |
2 |
|
Today™ |
2,319 |
2 |
|
Rogaine™ |
2,224 |
2 |
|
1,988 |
2 |
|
|
Mevacor™ |
1,940 |
1 |
|
Biaxin™ |
1,661 |
1 |
[† Excludes Novo Human insulins, Lilly Humalog,, NovoRapid, Lantus and all other synthetic insulins and all natural insulins.]
Top of Page
DRUG
CLASSES STRATIFIED BY HEALTH PROFESSIONALS OR
CONSUMERS,
TYPE OF REPORT, AND YEAR
Table
5 shows the top-five ranked drug classes
associated with suspect drugs, stratified by
whether the initial
reporter was a health
professional or consumer, the type of report,
and year the case was computerized into the
FDA
postmarket ADE database.
1995
Data. In 1995, there were 141,578 drug class
mentions where type of initial reporter and type
of report
were known. For consumers, only two of
the top-five ranked drug classes were common to
all report types: central
nervous system agents
and hormones and synthetic substitutes. For
health professionals, there were four drug
classes of the top-five ranked drug classes
common to all report types: central nervous
system agents, antineoplastic
agents,
anti-infective agents, and cardiovascular drugs.
The only drug class in the top-five ranked drug
classes
common to both consumers and health
professionals across report types was central
nervous system agents.
Trend
data from 1993 to 1995 show that only one drug
class in the top five-ranked drug classes was
common to both consumers and health
professionals as well as all report types for
all years: central nervous system
agents.
Captioned 3 page Table 5 is missing from the http://www.fda.gov/medwatch/safety/ar95.pdf pages.
Top of Page
ROUTES OF SUSPECT DRUGS
Table
6 presents the top-10 ranked routes of
administration associated with the suspect
drugs. There were
125,726 routes mentioned in
conjunction with the 130,950 postmarket ADE
cases. About three-fifths of the route
mentions
noted the oral route of administration.
|
Table 6. Postmarket ADE Reports by Top-10 Ranked Routes of Administration of Suspect Drugs: 1995 |
||
|
|
N |
% |
|
All Routes |
125,726 |
100 |
|
Oral |
77,75% |
62 |
|
Intravenous |
13,648 |
11 |
|
Subcutaneous |
9,534 |
8 |
|
Topical |
6,101 |
5 |
|
Intramuscular |
4,444 |
4 |
|
Vaginal |
3,426 |
3 |
|
Transdermal |
2,568 |
2 |
|
Inhalation |
2,266 |
2 |
|
Opthalmic |
1,721 |
1 |
|
Nasal |
1,014 |
1 |
Top of Page
ABATEMENT OF ADVERSE EVENT
For
the 153,842 suspect drug mentions, 74% (113,921)
had an answer to the question of whether the
adverse
event abated after the suspect drug was
stopped or the dose was reduced. Figure 7 shows
the distribution of
responses. Thirty percent
(34,433) of these 113,921 abate mentions
indicated a positive dechallenge
("Yes"
response).

For
the 153,842 suspect drug mentions, 70% (107,439)
had an answer to the question of whether the
adverse
event reappeared after reintroduction of
the suspect drug. Figure 8 shows the
distribution of responses. Four
percent (4,635)
of these 107,439 reoccur mentions indicated a
positive rechallenge ("Yes" response).

Top of Page
BODY SYSTEMS
There
were 140,182 body system mentions associated
with the adverse events of the 130,950
postmarket
ADE cases. The distribution of these
mentions across the 12 body systems is presented
in Figure 9. Four body
systems each had ³10% of the 140,182
body system mentions: body as a whole (systemic
adverse events) -
32%, nervous and skin and
appendages systems - each with 12%, and
digestive system - 10%.

Top of Page
ADVERSE EVENTS
Table
7 shows the top-10 ranked adverse events
reported with the 130,950 postmarket ADE cases.
The top
ranked ADE was "no drug
effect"; 10% of the ADE cases reported this
event. Metrorrhagia (2% of ADE cases), of
course, would be female associated.
|
Table 7. Postmarket ADE Reports by Top-10 Ranked Adverse Events: 1995 |
||
|
|
N |
% |
|
Adverse Event |
130,950 |
100 |
|
No Drug Effect |
13,416 |
10 |
|
Rash |
3,036 |
2 |
|
Application Site Reaction |
2,978 |
2 |
|
Aggravation of Existing Reaction |
2,757 |
2 |
|
Headache |
2,525 |
2 |
|
Metorrhagia |
2,354 |
2 |
|
Uticaria |
2,291 |
2 |
|
Alopecia |
2,021 |
2 |
|
Allergic Reaction |
1,924 |
1 |
|
Device Migration |
1,498 |
1 |
Top of Page
DRUG
CLASSES ASSOCIATED WITH
BODY SYSTEM
ADVERSE
EVENTS
Table
8 presents the four body systems comprising the
most adverse events, each of which has been
cross-
tabulated by its top-five ranked suspect
associated drug classes3. Central
nervous system agents and hormones
and synthetic
substitutes held the top-two ranks,
respectively, for all four body systems. Two
other drug classes were
in the top-five ranks
for all four body systems, cardiovascular drugs,
and anti-infective agents.
|
Table 8. Top-4
Ranked Body Systems with their
Respective |
|||
|
Body System |
Suspect Drug Class |
N |
% |
|
Body as a Whole |
All |
44,257 |
100 |
|
|
Central Nervous System Agents |
12,499 |
28 |
|
|
Hormones & Synthetic Substitutes |
5,074 |
11 |
|
|
Cardiovascular Drugs |
4,478 |
10 |
|
|
Anti-infective Agents |
4,074 |
9 |
|
|
Atineoplastic Agents |
2,689 |
6 |
|
Skin & Appendages |
All |
17,284 |
100 |
|
|
Central Nervous System Agents |
4,148 |
24 |
|
|
Hormones & Synthetic Substitutes |
2,294 |
13 |
|
|
Anti-infective Agents |
1,907 |
11 |
|
|
Skin & Mucous Membrane Agents |
1,816 |
10 |
|
|
Cardiovascular Drugs |
1,654 |
10 |
|
Nervous |
All |
16,930 |
100 |
|
|
Central Nervous System Agents |
4,421 |
26 |
|
|
Hormones & Synthetic Substitutes |
3,202 |
19 |
|
|
Cardiovascular Drugs |
1,966 |
12 |
|
|
Anti-infective Agents |
1,578 |
9 |
|
|
Atineoplastic Agents |
1,242 |
7 |
|
Digestive |
All |
13,333 |
100 |
|
|
Central Nervous System Agents |
4,131 |
31 |
|
|
Hormones & Synthetic Substitutes |
2,234 |
17 |
|
|
Cardiovascular Drugs |
1,199 |
9 |
|
|
Anti-infective Agents |
1,144 |
8 |
|
|
Atineoplastic Agents |
838 |
6 |
3 See Previous
--