BARRIERS TO DNA BASED DRUGS
INTRODUCTION
Ex vivo gene therapy approaches are
predominantly concerned with gene delivery barriers presented by the cell
itself. These intracellular barriers can include the plasma membrane, the
endosome, or the nuclear membrane. For sustained expression, maintenance of the
transgene within the nucleus might also be considered a barrier. In vivo
gene therapy and, to some extent, ex vivo therapies, must be concerned
not only with intracellular barriers but also with extracellular
barriers. Extracellular barriers, such as those posed by a specific tissue
or the immune system, can greatly diminish the effectiveness of in vivo
gene delivery.
I. Intracellular barriers.
The plasma membrane is the first obstacle to be overcome in
delivering genes into a cell. Our previous discussions on gene transfer vectors
explored a variety of approaches used to deliver genes into a cell. All of the
gene transfer vectors obtain entry into the cell either by endocytosis
or membrane fusion, but a detailed mechanism of entry for many gene
transfer vectors is still not fully understood. Clearly, viral vectors and
targeted vectors rely on binding to cell surface molecules prior to cellular
internalization. Liposomes, naked or complexed plasmid DNA must also interact
with the plasma membrane, but may do so in a non-receptor binding fashion prior
to cellular internalization. A schematic diagram of the potential intracellular
fate of a gene transfer vector is shown below.
Click on image to enlarge
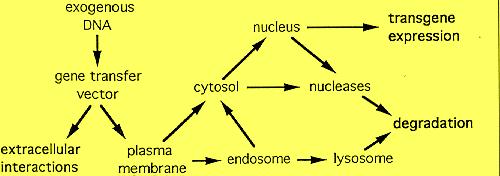
Once across the plasma membrane, gene transfer vectors must escape from the
endosome into the cytosome. Inability to escape from the endosome remains
problematic for many non-viral gene transfer vectors.
The last intracellular hurdle to gene delivery vectors is the nuclear
envelope. Nuclear entry of exogenous DNA appears to be regulated by the nuclear
pore complex(NPC).
The NPC accommodates both passive diffusion and active
transport. Molecules smaller than 10 nm in diameter or proteins less than 15
kDa, passively diffuse through the NPC into and out of the nucleus. Larger
macromolecules require active transport for nuclear entry. The exact mechanism
by which exogenous DNA passes through the NPC has not yet been determined,
although it may be very similar to the transport of proteins larger than 15 kDa
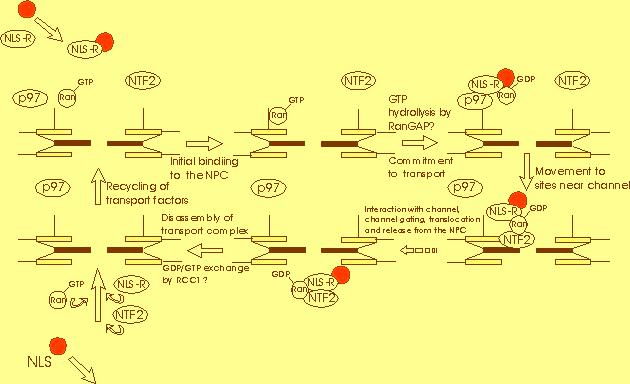
into the nucleus. The diagram below describes some of the steps involved in
protein transport into the nucleus (1). Nucleases present either within the
cytosol or the nucleus are obviously a concern of any DNA-based drug. The effect
of nucleases upon exogenous DNA can most easily be reduced by rapid integration
of the exogenous DNA.
Click on image to enlarge
II. Extracellular barriers.
Direct administration of gene transfer vectors into a patient, i.e., in
vivo gene therapy, must contend not only with intracellular barriers, but
also with extracellular barriers. Extracellular barriers presented to a gene
transfer vector predominantly fall into one of two categories, specific tissue
barriers or immunological barriers.
Tissue barriers can present a substantial hurdle for gene transfer
vectors. For example, any gene therapy aimed at treating CNS disorders must
contend with the blood-brain barrier. Currently, gene transfer vectors are
directly injected into the brain at or near the desired brain region.
Ultimately, development of a targeted CNS gene therapy agent which could be
administered intravenously would be preferred to a surgical procedure for
vector administration. Such a vector would need to be able to cross the
blood-brain barrier. Also, the nerve itself is somewhat resistant to gene
transfer by its very nature. Direct injection of a gene transfer vector into a
nerve is not only technically difficult, but usually harmful to the nerve. The
myelin sheath surrounding a nerve impedes the ability for vectors to transfer
genes into a nerve. These unique characteristics of the CNS may require a
vector possessing a natural tropism for the CNS, such as herpes simplex virus
(HSV) vectors, to be developed. Connective tissue barriers can also be a
substantial hurdle to gene delivery. The multiple connective tissue layers
found in muscle can diminish the spread and overall infectivity of vectors
administered intramuscularly. Epithelial cell linings can also interfere with a
vector's ability to infect deeper cell layers. Also, postmitotic cells will not
be infected by retroviral vectors. These few examples of potential tissue
barriers to gene transfer agents serve as examples of how a particular vector
system can be influenced by the target tissue or tissues surrounding it.
Immunological barriers of gene transfer agents are best understood
for viral vectors. Gene transfer vectors are susceptible to inactivation by
serum components are well as cellular and humoral immune responses.
Serum components can inactivate a variety of gene transfer agents.
For example, naked plasmid DNA can interact with a variety of positively-charged
serum proteins which impede its ability to deliver genes in vivo. Serum
proteases/nucleases also degrade both viral and non-viral vectors.
Complement inactivation is the predominant serum component responsible for
vector inactivation. One example, is the binding of complement component C1 to
MMLV surface proteins. Complement C1 binding initiates the classical complement
pathway, in an antibody-independent fashion, resulting in vector inactivation.
It is presumed that similar complement interactions can play a role in
inactivating other gene delivery systems. If complement interactions are not
observed the first time a vector is administered but antibodies against the
vector are formed, future administration of the vector will have to contend with
the binding of circulating antibodies and subsequent complement inactivation.
Cellular immune responses against recombinant adenoviruses has been
observed following intramuscular injection. Lymphocyte infiltrates have been
observed as early as 24 hours after adenoviral vector administration. CD8+
cells were also observed near the injection site. These cellular-mediated
responses are thought to be triggered by the expression of the viral proteins
from the regions encoded by the recombinant vector genome. Cellular-mediated
responses result in the elimination of those cells which were expressing the
transgene.
Antibodies against both retroviral and adenoviral vectors have been
observed following in vivo administration. In some cases these
antibodies existed prior to vector administration. There appear to be a
multitude of antibodies circulating within primate serum that interact with
retroviral coat proteins and envelope components. Currently, hurdles posed by
the immune system can be reduced through the use of immunosuppressant drugs.
Tolerance, a state of specific immunological unresponsiveness, against
viral vector proteins has been proven to be an effective means to overcome these
hurdles in experimental models but may prove difficult to reproduce in clinical
settings.
The degree to which intra- and extracellular barriers interfere with gene
delivery is dependent upon the vector system and the target tissue. The unique
characteristics of each vector system and the barriers it would encounter
represent the growing frontiers of new vector development. This has also led to
the investigation of the pharmacokinetics and pharmacodynamic parameters of
DNA- based drugs.
IDEAL GENE TRANSFER VECTOR
| Insert size | One or
more genes | | Titer | High concentration/ stable end
product | | Targeted | Yes,
target either entry into specific cells or limit expression to target cells |
| Immune Responce | None, safe for
recipient and enviroment | | Stable |
Yes, free of insertional mutangenesis |
| Production | Easy / reproducible |
| Regulatable | Yes,
levels of transgene expression can be up or down regulated as needed.
|
| 


