CYBERMEDICS
© 1999, Venkatesh.K.S
 MAJOR TRANSPLANT SURGERIES
MAJOR TRANSPLANT SURGERIES
TABLE OF
CONTENTS
INTRODUCTION
ORGAN DONATION
Live donation of organs or tissues is an established procedure today, especially in the fields of Kidneys, liver etc.Persons with two normally funcioning Kidneys may donate one of them, the risk of mortality being 1 in 3300 in a study conducted, with virtually no risk to the long term health of the donor.Live donation of unpaired organs has obvious difficulties,but successful live donation of segments of the liver and bowel has been undertaken, usually into childern from patients, although the safety of the procedure is still controversial.
CADAVERIC ORGAN DONATION
In most countries of the world the principal source of the organs for transplantation is cadaveric donation.The legal, ethical and religious principles vary, but many countries accept the concept of brain death which allows organ donation from beating heart donors.The donor must be carefully resuscitated before organ donation, the exact requirements depending on the cause of the death, but reversal of dehydration is commonly required, as are inotropes to maintain cardiac and urine output.The additional treatment with hormones such as thyroxine is controversial.Multi-organ donation should the rule, although there are practical age restrictions for use of livers (<60 yrs) and hearth (<50 yrs), and although renal dose dopamine is acceptable, higher doses of inotropes cause poor function of the heart after transplantation.Donor criteria are particularly stringent for lung donation, where pathological conditions such as infection is a frequent contraindication.
PRINCIPLES UNDERLYING ORGAN DONATION PROCEDURES
- Dissection of the organs to be removed to allow safe rapid removal after perfusion in situ.
- Dissectino of the main vessels to allow replacement of large bore cannulae, while maintaining blood flow to the organs.
- Ligation of accessible arterial branches not required for organ perfusion, to minimize loss of perfusion pressure.
- Start perfusion of the organs with cold perfusate at the moment the blood supply is interrupted.Additional surface cooling with iced saline packs or fluid is helpful.
- Removal of the organs, either en bloc or by careful dissection followed by flushing with perfusion fluid on the table.
- Removal of lymph nodes and spleen for tissue typing
- Careful suturing of the body and ensuring that cleaning and appropriate last rites are undertaken.
The perfusion fluids for organ donation vary for different organs and the constituents also vary considerably.It must be noted that they are not safe for organ perfusion in the living body, because many have a high potassium content.
The organs are packed in atleast 3 sterile plastic bags, then stored on ice for transport.COld pulsatile perfusion systems employed by certain centres appears to prolong the acceptable cold-ischaemia time.The duration for which an organ may be safely kept before transplantation varies depending on the organ, but is shortened if there is added warm ischaemia or if the donor is old.The viability of the organ beyond this safe-time then progressively deteriorates, although the consequences for some organs, such as the heart are more serious.
CHOOSING THE RECIPIENT
Recipients now, are no longer restricted to young, relatively fit patients.This applies to patients aged>70yrs also.All patients should be given a frank explanation of the procedure, the side effects and the risk to benefit ratios.The choice of the recipient must take into account the blood group of the donor, the HLA match and how long the recipient has been watching.Other clinical and virological aspects may also be considered.
LIVER TRANSPLANTATION
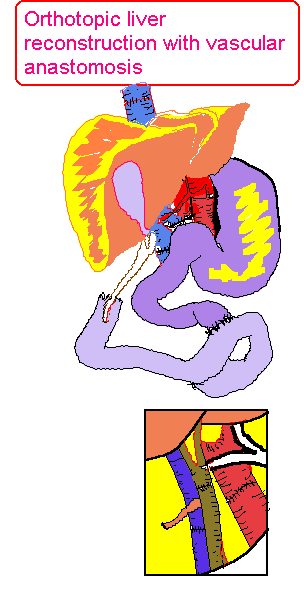 |
ORTHOTOPIC LIVER CONSTRUCTION WITH
VASCULAR ANASTOMOSIS |
 |
VENOUS BYPASS FROM THE VENACAVA
AND PORTAL SYSTEM TO THE SUPERIOR VENOUS SYSTEM |
 |
FUNCTIONAL DIVISIONS OF THE LIVER
AND THE SEGMENTS |
Liver transplantation has grown from a largely
experimental procedure, first carried out 35yrs ago, to a well established
treatment option for patients with advanced liver disease. Complete knowledge
of the surgical anatomy related to the liver is essential for both the
donor harvesting operation and the recepient operation.The shortage of
paediatric donors has resulted in the rapid development of innovative surgical
techniques where adult donor grafts are reduced in size to two or four
segments to implant into children.Starzl performed the first human liver transplantation in 1963.The early cases were all unsuccessful, but a further series by Starzl in 1967 paved the way to current success.The operative technique and degree of difficulty of liver transplantation is affected in a major way by the cause of liver failure and particularly by the presence of adhesions from previous surgery.In chronic cirrhosis, these adhesions can become extremely vascular because of portal hypertension, making the dissection and removal of the liver before transplantation one of the most difficult surgical procedures.
For this reason it is important that patients likely to come to eventual liver transplantation should not be subjected to other surgery unless the procedure is life-saving.If surgery is absolutely necessary
(for eg:- to transect the oesophagus for bleeding varices unresponsive to other therapy), then avoiding anterior approaches near the porta hepatis, for example by a left flank, approach is useful.
In parallel with the difficulty of surgery, anaesthesia of patients for liver transplantation is equally or more critical to the outcome, involving the control of wild swings in virtually every physiological system.These changes include circulatory volume depletion
as blood is lost, major stress on cardiac function as the venous return to the heart is halved during venacava cross clamping, changes in pH and electrolytes due to influx of metabolites and preservation fluid from the new liver, and major defects of coagulation, temparature control and glucose homeostasis.To control these changes requires an experienced anaesthetist team to undertake careful pre-operative assessment, particularly to exclude or assess any complicating defect of other organs, and intensive monitoring during the operation with adequate technical and laboratory backup close at hand.
DONOR LIVER REMOVAL
The conventional textbook description of a single
hepatic artery arising from the coeliac trunk is seen in only 60-65% of
cases, with anatomical variations being present in over one third of the
cases.The commonest variations are a replaced or accessory left hepatic
artery from the left gastric artery (20%), and a replaced or accessory
right hepatic artery arising from the superior mesentric artery (15%),
running posterior to the common bile duct.Occasionally both variants may
be present together (5%), or the entire hepatic arterial supply may be
derived from the superior mesenteric artery ro from a common coeliacomesenteric
trunk.Identification of the arterial supply is essential prior to perfusion
to cool and preserve the donor organs. To remove the liver from the donor,
the common bile duct is divided just above the pancreas, the gallbladder
incised and the bile flushed out prior to cooling, to prevent bile-induced
epithelial injury. After cross-clamping the aorta above the level of the
coeliac axis, cooling of the liver is achieved by portal venous and aortic
perfusion with the University of Wisconsic solution (UW) at 4*C.The liver
retrieval is completed, preserving an adequate length of inferior venacava
(IVC), the coeliac trunk with an aortic patch, and a suitable length of
portal vein. In addition, the common and external iliac arteries and veins
are retrieved in the event that a vascular reconstruction is necessary,
eg. because of portal vein thrombosis, or arterial vascular reconstruction.The
graft is then preserved for upto 18 hrs in UW at a temparature between
0-4*C.
RECIPIENT OPERATION
The use of UW has permitted safe prolonged storage
times, so that this operation is usually performed as a semi-elective procedure
during the day.The removal of the recipient'd diseased liver is usually
undertaken in the presence of portal hypertension, often with previous
biliary or portal surgery, and occasionally with additional technical problems
such as portal vein thrombosis or extensive varices.The structures in the
porta hepatis are systematically divided close to the liver hilum, the
hepatic artery, the common hepatic duct and the portal vein.Most centres
make use of a venovenous bypass in adults during the anhepatic phase, where
blood is pumped from the portal and femoral vein to the axillary vein;its
advantages include decompression of the clamped IVC and portal system,
providing adequate circulating blood volume and venous return, thereby
allowing for a more controlled anhepatic phase. Next the IVC is identified
below the liver and the left and right triangular ligaments are divided
and the bare area of the liver is dissected off the diaphragm.The suprahepatic
IVC is then dissected and encircled.The hepatectomy is completed after
placing supra- and infrahepatic IVC clamps.An alternative technique called
the 'piggy-bank' technique leaves the entire recipient IVC in place, with
the ligation of the individual short hepatic veins, particularly from the
caudate lobe. After achieving full control of bleeding, the new liver,
which has been dissected and prepared on a sterile trolley, is implanted
into the recipient: the upper IVC anastomosis first, the lower IVC next
followed by the portal vein using a continuous vascular suture. The UW
within the liver is next washed out.The graft is then reperfused with blood
via the portal vein.The arterial anastomosis is usually made between the
donor and recipient common hepatic arteries.In the event of a donor left
hepatic artery from the left gastric, no reconstruction is necessary,the
coeliac trunk being used for the anastomosis.A donor right hepatic artery
arising from the superior mesenteric artery requires some additional reconstruction,
most commonly this is anastomosed to the donor splenic artery stump.Not
infrequently, especially in patients undergoing transplantation for hepatic
artery thrombosis, it is often impossible to achieve an adequate arterial
inflow from the coeliac trunk, and a donor iliac artery aortic conduit
is constructed from the recepient infrarenal aorta to the donor hepatic
artery.Portal vein thrombosis is no longer a contraindication to liver
transplantation, and successful portal revascularization can be obtained
by thrombectomy, dissection posterior to the pancreas downt to healthy
portal vein, use of large collaterals, eg-left gastric vein, or by means
of a donor iliac vein graft from the recepient superior mesenteric vein
to the donor portal vein.
REDUCED SIZE LIVER TRANSPLANTATION
The majority of children with liver disease present
this condition in infancy and early childhood.It is in this group that
there is a great shortage of donor organs.Over the past decade, many groups
have shown that it is safe to implant reduced size grafts, with a reduction
in waiting time, fewer deaths on waiting lists, and with no increase in
complications related to the reduction process.These techniques allow a
donor-recipient weight discrepancy of upto 10:1, when only segments 2 and
3 are transplanted.The back-table reduction operation entails a meticulous
hilar and intrahepatic vascular and biliary dissection, dissection of the
left hepatic vein, and the parenchymal resection just to the right of the
falciform ligament.In larger children or when the discrepancy is less than
4:1, the right lobe (segments 5-8) may be utilized. The splitting of a
single donor liver into right and left lobes, thus benefitting two recipients,
is also an established procedure,where the vessels to one of the two halves
have to be lengthened using donor vessels.A few centres have gone one step
further, in an effort to further reduce the waiting period, to partial
transplants from living relations, where segments 2 and 3 of a parent's
liver are transplanted into a child.Over 100 such transplants have been
performed worldwide, particularly in the United States and Japan, with
low donor morbidity and excellent short and longterm results. In conclusion,
liver transplantation has developed into a major effort to support patients
with advanced liver disease.Although the techniques have been standardized,
it remains a difficult and complex procedure. A complete knowledge of the
anatomic variations in arterial supply,venous anatomy, as well as the segmental
anatomy of the liver, is an essential prerequisite to developing surgical
skills for this form of surgery.
POST-OPERATIVE CARE
After liver transplantation, the initial management follows standard intensive care, continuing the monitoring and attempted homeostasis started during anaesthesia.About a quarter of the patients will show rapid return to normality of all biochemical and physiological derangements, leading to weaning from the ventilator and return to the ward within 3 days.However, most patients will show some degree of early graft dysfunction, mostly as a result of cholestasis related to preservation or predonation injury.These patients may remain unconscious and have circulatory, clotting and electrolyte disorders which require continuing support.Slow recovery is to be expected in most of these cases, but the development of severe complications must always be excluded, as they require urgent action.
The development of rejection is less frequent than after kidney transplantation, usually coming on from 7 days onwards.Rejection usually presents as increasing graft dysfunction and must be distinguished from ischaemia, biliary obstruction and sepsis.Ultra sonography with color duplex scanning has greatly eased the diagnosis, and biopsy of liver helps distinguish rejection from infection.In the long term, the complications of immunosuppression are a problem, as after kidney transplantation, but one encouraging feature of liver transplantation is that there is less tendency to lose grafts late after the operation from chronic rejection (which usually manifests itself as 'vanishing bile duct syndrome' when it occurs).
SURGICAL COMPLICATIONS AFTER LIVER TRANSPLANTATION
COMPLICATIONS
- Rejection
- Haemorrhage
- Portal thrombosis
- Hepatic artery thrombosis
- Bile leak
- Cholangitis
- Biliary stricture
|
FEATURES
- Poor graft function
- Oliguria,CVP,Low BP
- Graft non-function
- Delayed liver necrosis
- Peritonitis
- Septicemia, reduced graft function
- Decreased graft function
|
DIAGNOSIS
- Biopsy
- Biopsy
- Clinical + Ultrasonography
- Duplex scan
- Duplex scan
- T-tube cholangiogram
- Biopsy, bile culture
- Ultrasonography
|
TREATMENT
- Immunosuppressive therapy
- Retransplantation if severe
- Correct clotting, re-exploration
- Re-transplantation
- Early exploration and thrombectomy
- Re-exploration
- Antibiotics
- Re-exploration or stenting
|
INDICATIONS AND OUTCOMES FOR ADULT LIVER TRANSPLANTATION
| | 5-year Graft Outcome |
| Alcoholic cirrhosis | variable* |
| Drug or toxin induced--hepatic failure | Good |
| Viral hepatitis | Fair |
| Chronic active hepatitis | Good |
| Primary biliary cirrhosis | Good |
| Hemochromatosis | Good |
| Budd-Chiari syndrome | Good |
| Hepatocellular and --Cholangiocarcinoma | Poor |
| Liver metastaseS | Very poor |
Good=60-90% 5-yr survival
Fair=30-60% 5-yr survival
Poor=10-30% 5-yr survival
*dependent on psychosocial factors etc
|
CURRENT RESULTS AFTER LIVER TRANSPLANTATION
These are influenced by the selection of patients transplanted as much as anyother factor.Excellent results can be obtained if recipients are confined to young patients with non-malignant disease, non-alcoholic cirrhosis, and fulminant hepatic failure is excluded; better than 90% of 1-year survival can be expected.To some extent the current shortage of donors justifies this selection approach.The challenge is to transplant the less favourable group, particularly those with fulminant liver failure where the longterm outcome of successful transplantation may be expected to be excellent.
PANCREATIC
TRANSPLANTATION
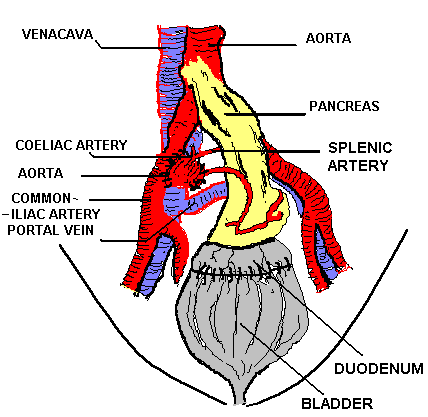 |
PANCREATICO-CYSTOSTOMY
WITH DUODENAL CONDUIT |
 |
PANCREATICO-CYSTOSTOMY
WITH CUT SURFACE OF PANCREAS ANASTOMOSED TO BLADDER |
Pancreatic transplantation is indicated for the treatment
of insulin dependent diabetics who suffer end-stage renal failure due to
diabetic micro-angiopathy;in these cases a kidney is transplanted into
the same recipient, usually from the same cadaveric donor.A pancreas may
also be transplanted alone in an unstable diabetic in whom renal or other
end-organ failure may be anticipated in the near future.The major technical
challenge has been to develop a method of drainage of the exocrine pancreatic
secretions and to prevent a pancreatic fistula.To achieve these ends, a
roux-en-y loop of jejunum has been used in the past, but the technique
of pancreaticocystostomy is now much more popular.The pancreas is always
transplanted into the pelvis, either within or outside the peritoneal cavity.In
this position, the pancreas can be drained into the bladder or into the
smallbowel loop, either using a duodenal conduit, or by anastomosing the
cut surfaces of the pancreas to the fundus of the bladder.The bladder anastomosis
offers the technical advantage of being an easier operation, and the function
of the gland may be measured by regular measurement of urinary amylase.In
immunosuppressed diabetics, where healing is impaired, pancreaticocystostomy
seems the safest and technically most successful operation.
OPERATION OF CADAVERIC PANCREATECTOMY
Following the diagnosis of brain death, the pancreas
is dissected free of the transverse mesocolon, and the attachments to the
greater curvature of the stomach, including the short gastric vessels,
are divided.The splenic vessels are tied at the pancreatic tail and the
spleen removed, and the splenic and coeliac arteries, including the superior
pancreatico-duodenal artery, are prepared for anastomosis to the recipient
artery.If the whole organ is to be transplanted, the duodenum is divided,
and the superior mesenteric trunk and the inferior pancreatico-duodenal
artery are preserved.Since the liver will also frequently be taken for
purposes of transplantation, the viscera are reperfused throught the aorta
with a precooled preservation solution and are excised.A patch of aorta
bearing the superior mesenteric and coeliac arteries may be removed, together
with a length of portal vein for anastomosis to recipient vessels.'Jump
graft' using sections of iliac artery and vein may be needed to provide
tension free anastomoses with the recipient's circulation.
RECIPIENT OPERATION
The coeliac and the superior mesenteric arteries
are anastomosed to the external iliac artery, and the portal vein to the
external iliac vein using a jump graft if necessary.Angulation must be
avoided to avoid a high-risk of postoperative thrombosis.The cut pancreatic
surface, or the duodenal conduit, are anastomosed to the vault of the bladder.
KIDNEY TRANSPLANTATION
Transplantation of the kidney is now recognized as
the definitive treatment of end-stage renal failure.Although kidneys from
compatible living relatives may be used, the commonest source of donor
organs are patients who die during intensive care for lethal head injury
or intracranial haemorrhage.Dramatic advances in the pharmacology of immunosuppression
have led to greater longterm success in graft function and patient survival:rejection
of the graft is preventable in some cases and is successfully treated in
the majority of cases when it occurs.70% graft survival after cadaveric
renal transplantation is now reported five years after the operation described
here.
For technically successful transplantation of
the kidney to be carried out, the following criteria must be met:
-
The donor Kidney must be in good physiological condition
to withstand a period of shortage without a blood supply prior to its transplantation
into the recipient.The development and recognition of the criteria of brain
death enable the dissection of the donor kidney in the brain-dead cadaver
whilst the circulation is maintained.The time that the kidney is exposed
to ischaemia whilst it is still warm can therefore be shortened to 2-3
minutes.
-
The renal artery & vein and the ureter must be
preserved during the donor nephrectomy in such a way as to allow the anastomosis
of the vessels to the recipient circulation.
-
The artery to the ureter (a branch of the renal artery)
must be preserved in order to avoid post-operative ureteric necrosis.
-
Following its removal, the kidney must be flushed
with a cold preserving solution (high osmolarity, with a high concentration
of potassium, calcium and magnesium) which minimizes tissue injury during
prolonged cold preservations in ice.
Provided these criteria are met, kidneys taken from
brain dead, heart-beating donors may be stored for periods upto 72 hours
and re-implanted with a good prospect of immediate function.
DONOR KIDNEY
The native human kidney lies in the retroperitoneal
space, deriving its blood supply from the aorta via the renal arteries,
and its venous drainage entering directly into the inferior venacava on
each side through the renal vein.Vascular anatomy may vary: usually the
renal artery is single, but renal arteries may be multiple in approximately
15% of cases.Each renal artery is an end artery (i.e. intrarenal anastomoses
with accessory arteries do not occur).Collateral venous anastomoses do,
however, occur within the renal substance, between the main renal vein
and accessory veins.From the surgical point of view, therefore, the establishment
of a complete blood supply to the donor kidney requires that all the accessory
arteries must be joined to the recipient's circulation, whereas accessory
veins may be ligated and the venous drainage established by anastomosing
the main renal vein to the recipient's venous system.The renal artery commonly
divides into two or three subsidiary branches near the hilum of the kidney,
and the inferior of these branches frequently gives rise to arterial twigs
which supply the upper third of the ureter, which must be preserved.
RECIPIENT OPERATION
The donor kidney need not be placed orthotopically
in its 'natural' position;indeed there are anastomical advantages to transplanting
kidney into the pelvis.The iliac fossa is anatomically receptive to a renal
transplant.The time-honoured technique invented by Murray utilizes an extra-peritoneal
approach to the iliac fossa which allows ready access to the iliac artery
and its branches, and the external iliac vein, to which the donor vessels
may be joined;the recipient bladder and ureter are nearby, enabling the
surgeon to achieve a satisfactory junction for urine to drain into the
bladder, using a small length of the upper ureter, with its own blood supply
deriving from the renal artery.
TECHNIQUE
The arterial anastomosis:
when the donor kidney is removed from a cadaver, a patch of aorta bearing
the renal artery of arteries greatly facilitates the anastomosis of the
patch to the external iliac artery (Fig I)
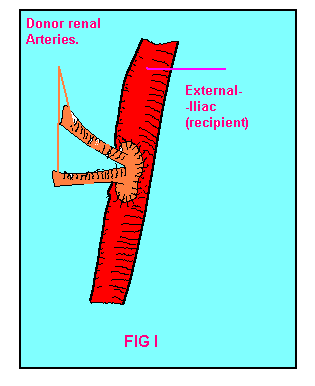 |
FIG-I Renal arteries
of donor joined to ext.iliac artery of recipient. |
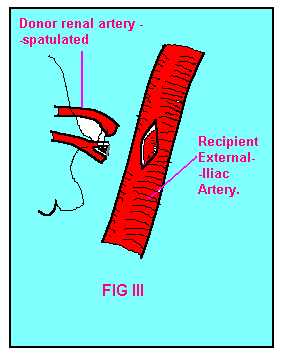 |
FIG-III Anastomosis
of renal arteries to ext.iliac artery. |
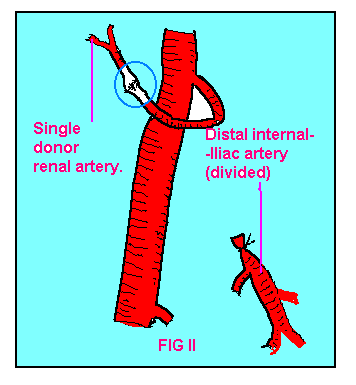 |
FIG-II. End-end anastomosis
of donor renal artery to int.iliac artery of recipient. |
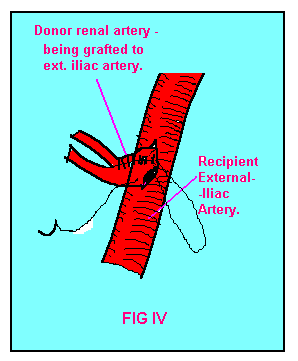 |
FIG-IV.Technique of
surgical anastomosis of renal arteries. |
Kidneys taken from living donors usually have a single
main artery.Under these circumstances, the main artery is best anastomosed
to the end of the internal iliac artery, after division of the distal end,
and reflection of its proximal end to enable junction to the renal artery
to take place (Fig II). If an inferior polar artery exists, it is commonly
inferior and may be joined end to end to the inferior epigastric artery.
Exceptionally two 'main' renal arteries are found which may have to be
spatulated (joined together side-to-side for a short distance to form a
common osteum) and then anastomosed conjointly to the side of the external
iliac artery (FIG III & IV). Small polar arteries less than 1 mm in
external diameter may be sacrificed provided that the area of cortex supplied
by such an artery does not exceed an area 3 cm in diameter.Failure to anastomose
bigger polar arteries than this risks necrosis of the cortex and calyceal
fistula with urine leak.
The venous anastomosis: The
biggest donor vein is chosen to join to the recipient vein, and other branches
may be sacrificed safely on account of the intrarenal venous anastomoses.Provided
that a suitable length of donor vein is available, the renal vein may be
joined safely to the external iliac vein.
two techniques have been developed to join the donor
ureter to the recipient urinary tract: implantation of the ureter into
the bladder and anastomosis of the donor ureter or renal pelvis to the
recipient ureter (ureteroureteral anastomosis).
IMPLANTATION OF THE URETER INTO THE BLADDER
On-lay technique:
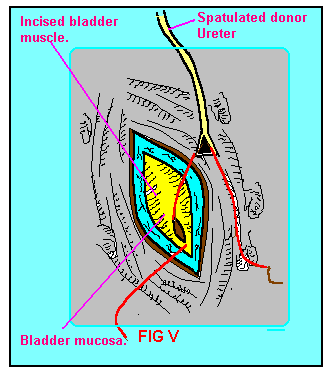 |
FIG-V. The on-lay technique
of Uretero-cystostomy. |
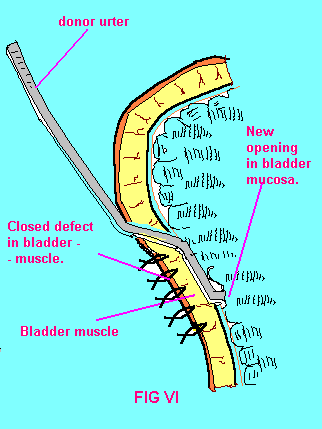 |
FIG-VI. Cross-section
of completed On-lay ureterocystostomy. |
the ureter is spatulated by incising one side of
its distal end 1 cm proximally, the bladder muscle is incised over its
supero-lateral aspect so that the mucosa bulges forward for a length of
3 cm; the lower 1 cm of the bladder mucosa is opened and is stitched with
an absorbable suture to the spatulated ureter (Fig V).An anti-reflux mechanism
is then created by approximating the bladder muscle over the distal end
of the ureter (FIG VI).
The 'ureteroneocystostomy' (anastomosis of the
ureter from within, using a wide opening in the bladder)
Here, the dome of the bladder is widely opened
and a tunnel is created throught the bladder muscle approximately 1.5cm
in length.
 |
FIG-VII. The 'ureteroneocystostomy'
seen in cross-section. |
The donor ureter is then drawn down through this
tunnel, spatulated and the ureteric mucosa is joined to the bladder mucosa
using interrupted absorbable sutures.This technique allows the ureter to
prolapse into the lumen of the bladder, forming a 'nipple' ureteroneocystostomy.After
healing, the bladder muscle which embraces the lower end of the ureter
acts as an effective anti-reflux mechanism.During contraction of the bladder
wall during micturition, the ureteric lumen is closed by contraction of
the bladder muscle, which prevents reflux up the donor ureter and thus
minimizes the risk of infection or obstructive uropathy (Fig VI). The dome
of the bladder is then closed to ensure a urine-proof junction.After on-lay
ureterocystostomy, or ureteroneocystostomy, a bladdder catheter is left
insitu for 5 days, to protect the bladder and ureteric anastomoses from
back pressure should post-operative urinary retention occur.
Uretero-ureteral anastomosis This
is an alternative to the ureterocystostomy techniques described above.The
donor ureter is divided 2-3 cms below the pelvi-ureteric junction and the
recipient ureter is dissected over a short distance, care being taken to
preserve blood supply.The proximal recipient ureter is ligated and divided.Both
donor and recipient ureters are reciprocally spatulated and joined together
using a single or interrupted layer of absorbable sutures.A plastic stent
may be inserted across this anastomosis leading from the donor renal pelvis
to the recipient bladder to prevent leakage of urine through the anastomosis
during the healing phase.
Good anatomical insight & surgical techniques
are essential to successful renal transplantation.Meticulous anastomosis
of blood vessels, enabling early endothelial healing, will minimize the
risk of vascular thrombosis and inevitable graft failure.The ureteric anastomosis
must also be sound to prevent urine leakage which in an immunosuppressed
patient recieving drugs to prevent rejection can lead to local infection
and septicemia.
EXTRAS MYHOME LINKS SEARCH











